Rep:Mod:zy1416
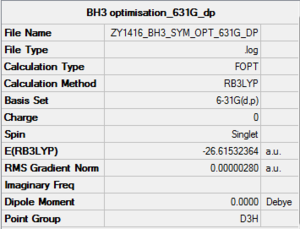
BH3
Optimisation Summary
B3LYP/6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000022 0.001800 YES RMS Displacement 0.000015 0.001200 YES
Frequency analysis log file media: ZY1416_BH3_FREQ.LOG
Low frequencies --- -2.2126 -1.0751 -0.0054 2.2359 10.2633 10.3194 Low frequencies --- 1162.9860 1213.1757 1213.1784
Optimised BH3 molecule |
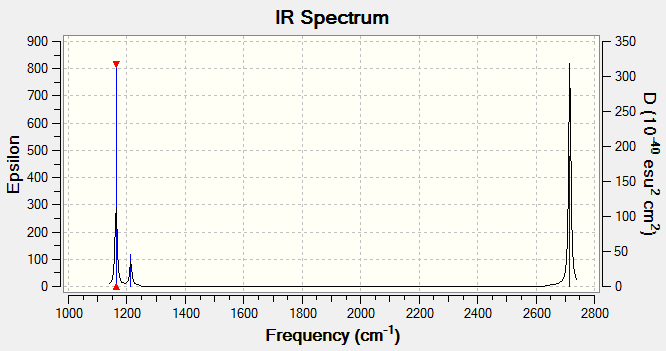
Vibratioal Spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2'' | yes | out-of-plane bend |
| 1213 | 14 | E' | very slight | bend |
| 1213 | 14 | E' | very slight | bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
Some of the vibrations are degenerate, and give rise to a single peak in the spectrum. The two bend vibrational modes are degenerate and the two asymmetric stretch vibrational modes are degenerate. While the symmetric stretch is IR inactive and will not give rise to a peak.
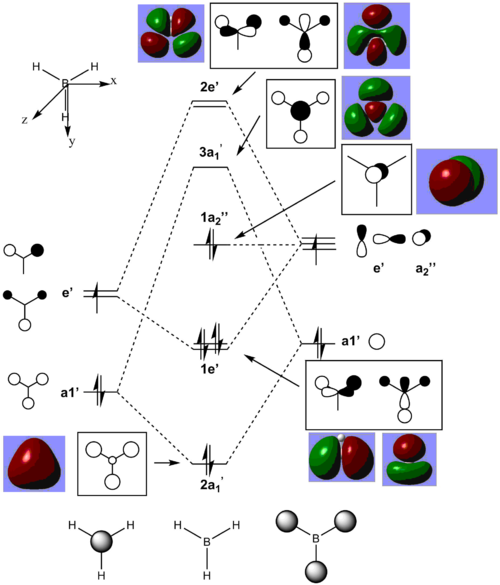
MO diagram
There are no significant differences between the real and LCAO MOs, meaning the qualitative MO theory is fairly useful and accurate.
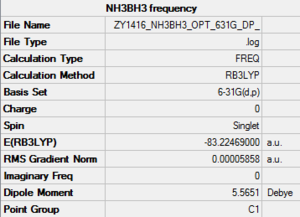
Association energies: Ammonia-Borane
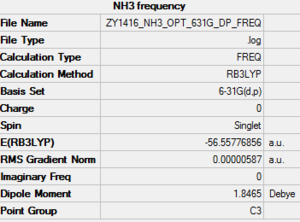
Key data of ammonia
B3LYP/6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency analysis log file is linked here Media:ZY1416_NH3_OPT_631G_DP_FREQ.LOG
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Key data of ammonia-borane
B3LYP/6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000540 0.001800 YES RMS Displacement 0.000296 0.001200 YES
Frequency analysis log file is linked here Media:ZY1416_NH3BH3_OPT_631G_DP_FREQ.LOG
Low frequencies --- -0.0006 -0.0005 0.0005 19.1547 24.7625 35.3188 Low frequencies --- 265.5547 632.2427 639.4905
Energy calculation
E(NH3)= -56.55777 a.u.
E(BH3)= -26.61532 a.u.
E(NH3BH3)= -83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -0.0516 a.u. = -135 kJ/mol
Ng611 (talk) 20:59, 17 May 2018 (BST) Correct calculation and good consideration given to the accuracy of the reported result.
The dissociation energy of B-N dative bond is 135 kJ/mol.
A B-N dative bond is weak since the dissociation energy of a C-C covalent bond is about 350 kJ/mol as mentioned in the wiki page.
Ng611 (talk) 22:56, 15 May 2018 (BST) I would say that the wiki page isn't a particularly good source to cite from. A textbook, databook, or paper would be better.
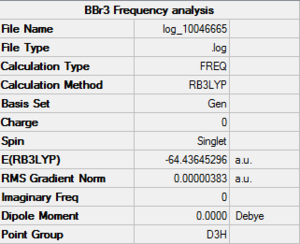
BBr3
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency analysis log file is linked here DOI:10042/202297
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Optimised BBr3 molecule |
Lewis acids and bases
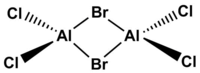
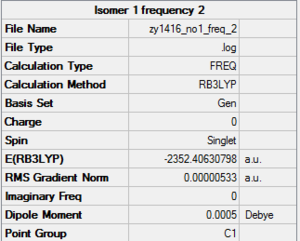
isomer with 2 bridging Br ions
Point group :D2h
Method: 6-31G(d.p.) for Al and Cl, LanL2DZ for Br
The point group is expected to be D2h but the symmetry was dropped to C1. There might be slight differences in the bond angle and bond length under the computational calculation which breaks the symmetry.
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.001209 0.001800 YES RMS Displacement 0.000501 0.001200 YES Predicted change in Energy=-1.592590D-08
Frequency analysis log file is linked here Media:Zy1416_no1_freq_2.log
Low frequencies --- -5.1842 -5.0672 -3.1595 -0.0021 0.0025 0.0030 Low frequencies --- 14.8210 63.2656 86.0733
isomer 1 |
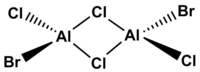
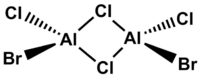
isomer with trans terminal Br and bridging Cl ions
Point group: C2h
Method: 6-31G(d.p.) for Al and Cl, LanL2DZ for Br
Again the point group of the optimised molecule is C1 so the molecule is not perfectly C2h when it has the lowest energy under calculation.
Item Value Threshold Converged? Maximum Force 0.000056 0.000450 YES RMS Force 0.000026 0.000300 YES Maximum Displacement 0.000575 0.001800 YES RMS Displacement 0.000330 0.001200 YES Predicted change in Energy=-5.888915D-08
Frequency analysis log file is linked here Media:Zy1416_no2_freq_2.log
Low frequencies --- -5.1102 0.0008 0.0030 0.0036 1.4834 2.0609 Low frequencies --- 18.1581 49.1067 73.0081
isomer 2 |
Monomer
Comparison between two isomers
The energy of the isomer with 2 bridging Br ions is -2352.40630 a.u. (5 d.p.)
The energy of the isomer with trans terminal Br and bridging Cl ions is -2352.41630 a.u. (5 d.p.)
The relative energy E(isomer 1)-E(isomer 2) = 0.01000 a.u. = 26 kJ/mol
So the second isomer has lower energy and is more stable. The bridging bonds are shorter than the terminal bonds, therefore larger bridging ions means greater repulsion which results in higher energy. And Br is a 3rd row element which is larger than Cl.
Calculation
Method: 6-31G(d.p.) for Al and Cl, LanL2DZ for Br
Item Value Threshold Converged? Maximum Force 0.000081 0.000450 YES RMS Force 0.000042 0.000300 YES Maximum Displacement 0.001588 0.001800 YES RMS Displacement 0.000974 0.001200 YES Predicted change in Energy=-1.810813D-07
Frequency analysis log file is linked here Media:Zy1416_monomer_freq.log
Low frequencies --- -0.0035 -0.0018 -0.0011 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
monomer |
The dimer is more stable the isolated monomer.
And the dissociation energy for the lowest energy conformer into 2 AlCl2Br is
= 2*E(AlCl2Br)-E(Al2Cl4Br2) = 2*(-1176.19013)-(-2352.41630) a.u.
= 0.03604 a.u. = 95 kJ/mol
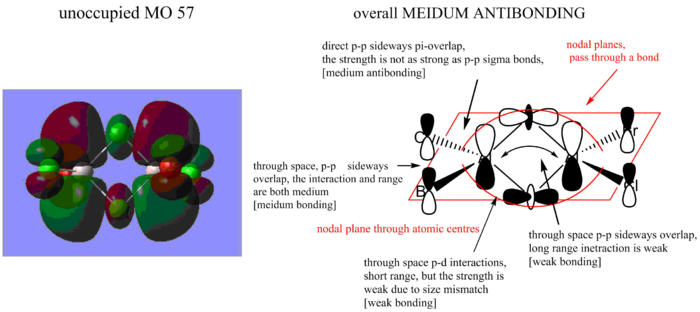
MO calculation of the isomer with trans terminal Br and bridging Cl ions
MO 1
MO 2
MO 3
Ng611 (talk) 21:01, 17 May 2018 (BST) Good LCAO analysis - interesting use of d-orbitals in MO3
Other isomers
symmetry: C2v
symmetry: C1
symmetry: C2h
Ng611 (talk) 22:59, 15 May 2018 (BST) A very solid report, well done. Remember to use citations from the literature wherever possible (as opposed to wiki pages, websites etc). Also remember to include all of the required information when performing calculations (you missed out a couple of jmol files). Your MO analysis was very good, well done.