Rep:Mod:zx616
Testing Molecule NH3
Molecule Name: NH3
Calculation Method: RB3LYP
Calculation Type: FREQ
Basis Set: 6-31G(d.p)
E(RB3LYP): -56.44397188 a.u.
Point Group: C3V
N-H Bond Length: 1.01798Å
H-N-H Bond Angle: 37.129°
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986282D-10
Optimization completed.
-- Stationary point found.
Ammonia Molecule |
The optimisation file is linked to here
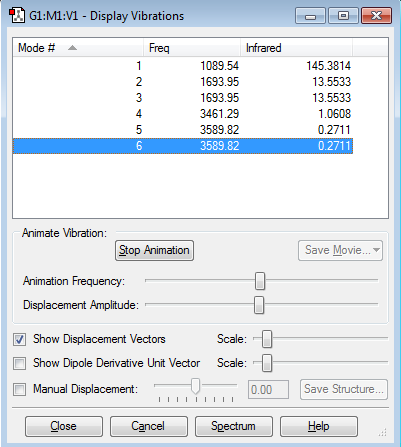
how many modes do you expect from the 3N-6 rule?
--6
which modes are degenerate (ie have the same energy)?
--Number 2 and Number 3; Number 5 and Number 6.
which modes are "bending" vibrations and which are "bond stretch" vibrations?
--Number 1,2 and 3 are "bending" vibrations and Number 4,5,and 6 are "bond stretch" vibrations
which mode is highly symmetric?
--Number 1 is highly symmetric
one mode is known as the "umbrella" mode, which one is this?
--Number 1
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
--4
Charges on atoms:
Nitrogen: -1.125e
Hydrogens: 0.375e
N2 Molecule
Molecule Name: N2
Calculation Method: RB3LYP
Calculation Type: FREQ
Basis Set: 6-31G(d.p)
E(RB3LYP): -109.52412868 a.u.
RMS Gradient Norm: 0.00000060 a.u.
Point Group: D*H
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401066D-13
Optimization completed.
-- Stationary point found.
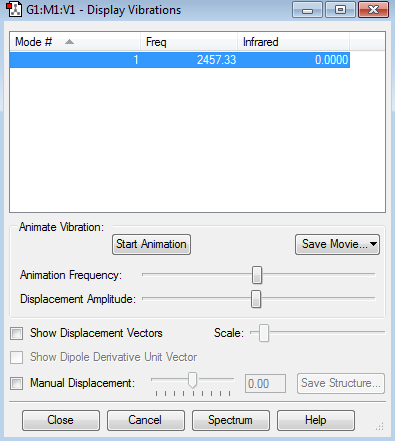
Nitrogen Molecule |
The optimisation file is linked to here
H2 Molecule
Molecule Name: H2
Calculation Method: RB3LYP
Calculation Type: FREQ
Basis Set: 6-31G(d.p)
E(RB3LYP): -1.15928020 a.u.
RMS Gradient Norm: 0.09719500 a.u.
Point Group: D*H
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
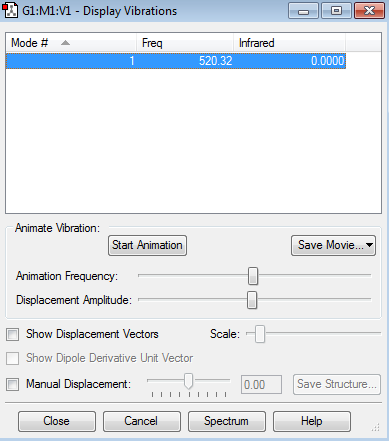
Hydrogen Molecule |
The optimisation file is linked to here
Reaction Energy
N2 + 3H2 -> 2NH3
E(NH3)=-56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)=-109.52412868 a.u.
E(H2)=-1.17853936 a.u.
3*E(H2)=-3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557907 a.u.=-146.47849401 kJ/mol
Conclusion: The ammonia product is more stable than gaseous reactants because the reaction is exothermic, therefore the product is at a lower energy level than the reactants.
Molecule of My Choice--Cl2
Basic Information:
Molecule Name: Cl2
Calculation Method: RB3LYP
Calculation Type: FREQ
Basis Set: 6-31G(d.p)
E(RB3LYP): -920.34853293 a.u.
RMS Gradient Norm: 0.01404387 a.u.
Point Group: D*H
Item Value Threshold Converged?
Maximum Force 0.000043 0.000450 YES
RMS Force 0.000043 0.000300 YES
Maximum Displacement 0.000121 0.001800 YES
RMS Displacement 0.000172 0.001200 YES
Predicted change in Energy=-5.277205D-09
Optimization completed.
-- Stationary point found.
Chlorine Molecule |
The optimisation file is linked to here
Charges of the atom:
Cl: 0.000e
This is because that Cl2 is a diatomic molecule, and the bonding is covalent, therefore the electrons are equally distributed around the molecule, leaving both atoms neutral.
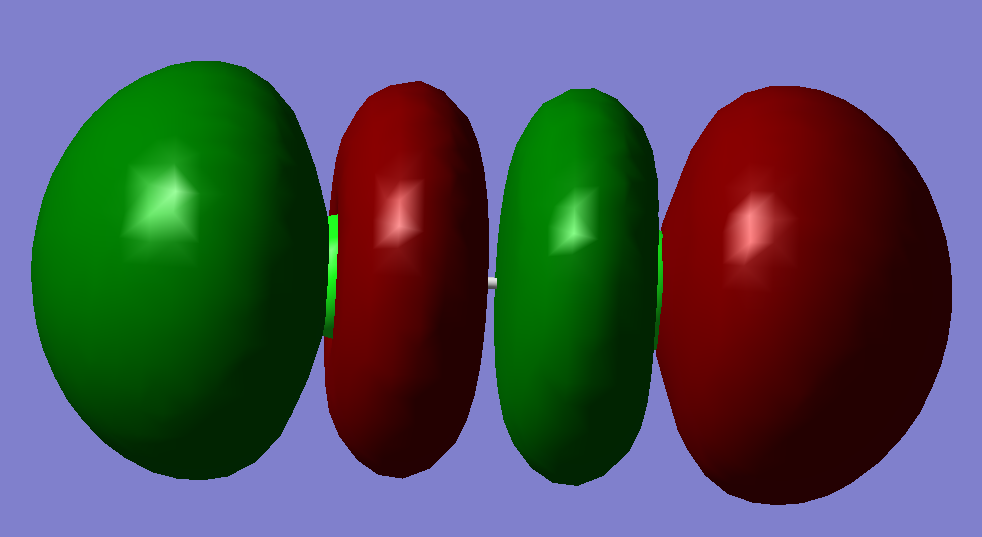
Molecular Orbitals Analysis:
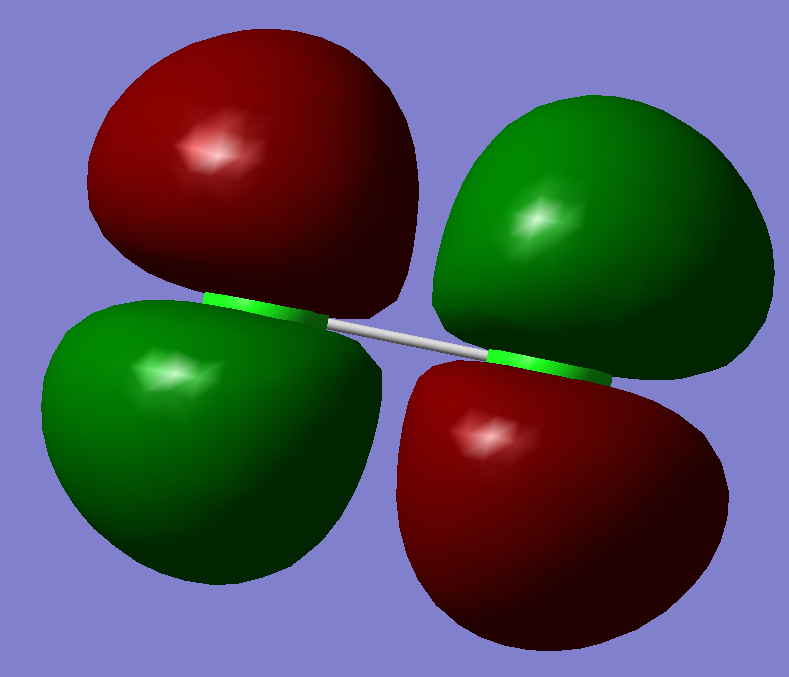
This is a sigma anti-bonding orbital. It is also the highest unoccupied molecular orbital. It has the highest energy of -0.14206 a.u.. It is likely to be formed by mixing two Pz orbitals.
This is a pi bonding orbital. It has an energy of -0.40695 a.u.. It also comes with another degenerate orbital that has the same energy. It is formed by mixing two Px orbitals. It is neither HUMO nor LOMO.
This is a pi anti-bonding orbital. It has an energy of -0.31361 a.u.. This orbital is an occupied orbital with high energy. It also comes with another degenerate orbital that has the same energy. These two anti-bonding orbitals has an opposite effect with the previous pi bonding orbitals therefore there is no net effect in pi bond, so there is no pi bonding in Chlorine molecule.
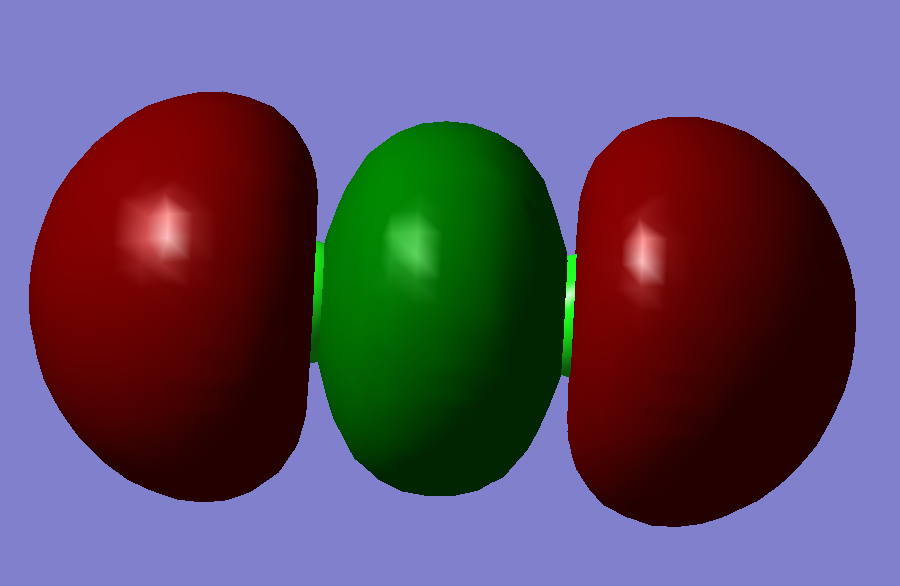
This is a sigma bonding orbital, and it is also the lowest energy occupied molecular orbital. It is formed by two Pz orbital mixing. It has an energy of -0.47392 a.u.. This is the only effective molecular orbital that contribute to the only sigma bond exists in Chlorine molecule.
This is a sigma anti-bonding orbitals. It has an energy of -0.77746 a.u.. It is formed by mixing two 3s orbitals. This is an occupied molecular orbital.