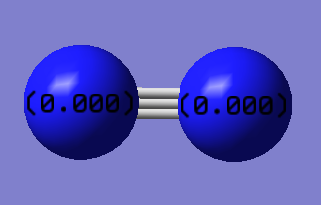Rep:Mod:zl4817
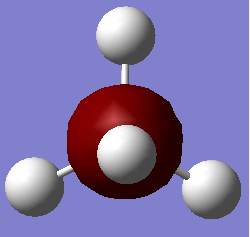
NH3 Molecule Information
Optimization Information
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -56.55776873 a.u.
RMS Gradient: 0.00000485 a.u.
Point Group: C3V
Geometry
N-H Bond Distance: 1.01798Å
H-N-H Bond Angle: 105.741°
Comments: Nitrogen is sp3 hybridized. N uses one 2s orbital and three 2p orbitals to form four sp3 hybrid orbitals, each forming a σ bond with one 1s orbital of hydrogen.
According to VSEPR theory, NH3 molecule adopts a tetrahedral pseudostructure (4 electron domains) while its real structure is trigonal pyramidal.
A perfect tetrahedral bond angle as predicted is 109.5°. However in a trigonal planar shape, the bond angle is slightly less than 109.5° as the lone pair of electrons repel the bond pairs of electrons more, pushing the bond pairs closer to each other, leading to decrease in bond angle. 3
The optimized bond angle calculated by Gaussian is in good agreement with the theoretic prediction.
Item Table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Jmol Dynamic Image
NH3 Molecule |
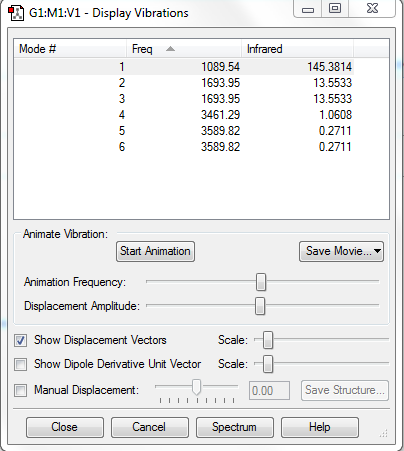
Frequency Analysis
Number of Modes Expected: 3N-6=6 (N=4; non-linear molecule)
Degenerate Modes: Modes 2 and 3: Frequency 1693.95cm-1; Modes 5 and 6: Frequency 3589.82cm-1
Bending Modes: Modes 1, 2 and 3
Stretching Modes: Modes 4, 5 and 6
Highly Symmetric Mode: Mode 4
Umbrella Mode: Mode 1
Number of bands expected to see in an experimental spectrum of gaseous ammonia: 2
Explanation: In theory, 4 bands would be seen, which have intensities of 145.3814 (from Mode 1), 27 (combined value for degenerate Modes 2 and 3), 1.0608 (Mode 4), 0.542 (combined value for degenerate Modes 5 and 6). These vibration modes are observable as these motions involve change in dipole moment. However, bands with intensities 1.0608 and 0.542 are too minor so they would be ignored. Hence, only 2 bands would be seen.
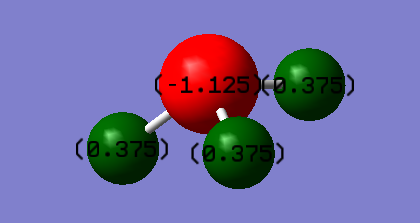
Atomic Charge
Charge on N atom: -1.125
Charge on H atom: 0.375
Magnitude of dipole moment: 1.8466 Debye
It is expected that nitrogen will have a negative charge while hydrogen having a positive charge, as nitrogen is more electronegative than hydrogen.
N2 Molecule Information
Optimization Information
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -109.52412868 a.u.
RMS Gradient: 0.00000060 a.u.
Point Group: D∞h
Geometry
N≡N Bond Distance: 1.10550Å
N≡N Bond Angle: 180°
Comments:
Nitrogen is sp hybridized. Each N atom uses one 2s orbital and one 2p orbital to form 2 sp hybrid orbitals. Each N uses one sp hybrid orbital to form a σ bond between them while the other sp hybrid orbital holds a lone pair of electrons. Each N uses two unhybridized 2p orbitals to form two π bonds.
By VSEPR, the molecule adopts linear shape for both pseudostructure and real structure. The bond angle is 180°. 3
Item Table
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Jmol Dynamic Image
N2 Molecule |
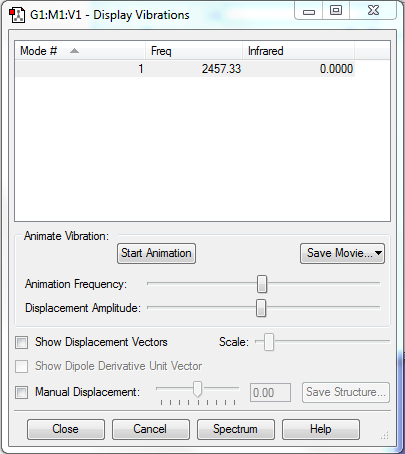
Frequency Analysis
Number of Modes Expected: 3N-5=1 (N=2; linear molecule)
Degenerate Modes: N/A
Bending Modes: N/A
Stretching Modes: Mode 1 with frequency 2457.33cm-1
Highly Symmetric Mode: Mode 1
Umbrella Mode: N/A
Number of bands expected to see in an experimental spectrum of gaseous ammonia: 0
Explanation: The vibration mode of molecule does not induce a change in dipole moment, hence it could not be observed in IR spectrum.
Atomic Charge
Charge on both N atoms: 0
Magnitude of dipole moment: 0 Debye
H2 Molecule Information
Optimization Information
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -1.17853936 a.u.
RMS Gradient: 0.00000017 a.u.
Point Group: D∞h
Geometry
H-H Bond Distance: 0.74279Å
H-H Bond Angle: 180°
Comments:
Each H atom uses its 1s orbital to generate a σ bond.
By VSEPR, the molecule adopts linear shape for both pseudostructure and real structure. The bond angle is 180°. 3
Item Table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Jmol Dynamic Image
H2 Molecule |
Frequency Analysis
Number of Modes Expected: 3N-5=1 (N=2; linear molecule)
Degenerate Modes: N/A
Bending Modes: N/A
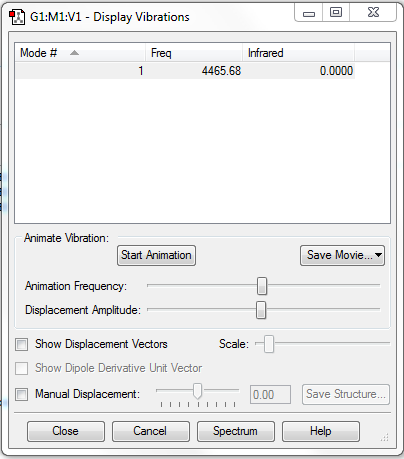
Stretching Modes: Mode 1 with frequency 4465.68cm-1
Highly Symmetric Mode: Mode 1
Umbrella Mode: N/A
Number of bands expected to see in an experimental spectrum of gaseous ammonia: 0
Explanation: The vibration mode of molecule does not induce a change in dipole moment, hence it could not be observed in IR spectrum.
Atomic Charge
Charge on both H atoms: 0
Magnitude of dipole moment: 0 Debye
Haber Process Reaction Energy Analysis
Reaction: N2 + 3H2 ⇌ 2NH3
Energy Calculations
E(NH3) = -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579070 a.u. = -146.48 kJ/mol (2.d.p.)
The gaseous reactant N2 is the most stable as it has the most negative (i.e. lowest) energy value. This is understandable considering the strong N≡N bond which requires a large amount of energy to overcome.
The overall energy change was a large negative value, which indicates that it is a highly thermodynamically favourable reaction. However in reality, in order to achieve optimized yield and reaction rate, we need to use a relatively high temperature (400–500 °C)1 and high pressure (150–250 atm)2 . Such conditions allow us to achieve a balance between high reaction rate and equilibrium largely lying towards the product. In addition, a Fe catalyst is also used to enhance the rate of reaction, although it does not alter the equilibrium position.
CH4 Molecule Information
Optimization Information
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -40.52401404 a.u.
RMS Gradient: 0.00003263 a.u.
Point Group: Td
Geometry
C-H Bond Distance: 1.09197Å
H-C-H Bond Angle: 109.471°
Comments:
Carbon is sp3 hybridized. Each C atom uses one 2s orbital and three 2p orbitals to form 4 sp3 hybrid orbitals. Each C uses one sp3 orbital to form a σ bond with the 1s orbital of H atom.
By VSEPR, CH4 adopts tetrahedral shape for both pseudostructure and real structure. 3
The optimized bond angle calculated by Gaussian is in good agreement with prediction by VSEPR.
Item Table
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Jmol Dynamic Image
CH4 Molecule |
Frequency Analysis
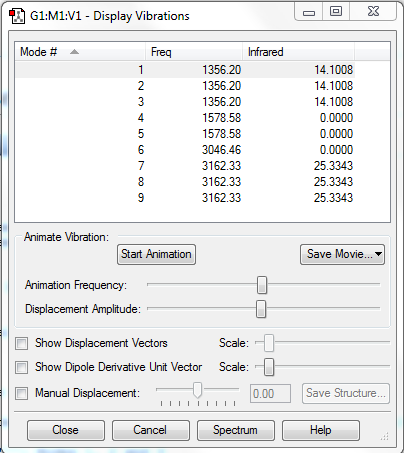
Number of Modes Expected: 3N-6=9 (N=5; non-linear)
Degenerate Modes: Modes 1, 2 and 3: Frequency 1356.20cm-1; Modes 4 and 5: Frequency 1578.58cm-1; Modes 7, 8 and 9: Frequency 3162.33cm-1;
Bending Modes: Modes 1, 2, 3, 4 and 5
Stretching Modes: Modes 6, 7, 8 and 9
Highly Symmetric Mode: Mode 6
Umbrella Mode: N/A
Number of bands expected to see in an experimental spectrum of gaseous ammonia: 2
Explanation:2 bands would be seen, which have intensities of 42.3024 (combined value for degenerate Modes 1, 2 and 3), and 76.0029 (combined value for degenerate Modes 7, 8 and 9). These vibrations can be observed as their motion induces a change in dipole moment. The vibration modes 4, 5 and 6 do not induce a change in dipole moment, hence they could not be observed in IR spectrum.
Atomic Charge
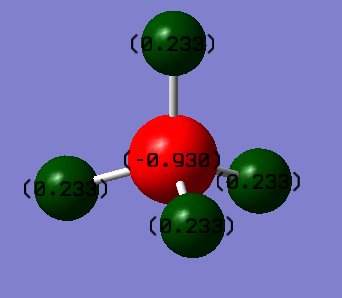
Charge on C atom: -0.930
Charge on H atom: 0.233
Magnitude of dipole moment: 0 Debye
It is expected that carbon will have a negative charge while hydrogen having a positive charge, as carbon is more electronegative than hydrogen.
Although C and H has different electronegativities i.e. each C-H bond is polar, the overall dipole moment is 0 as the dipole moments of each C-H bond cancel out with each other.
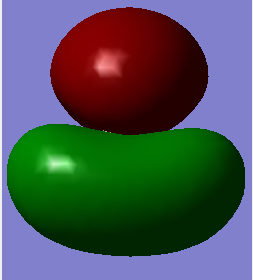
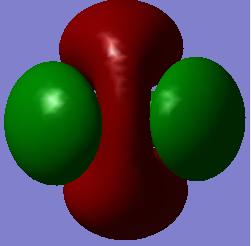
Molecular Orbital Analysis
MO 1
-Energy: -10.16707 a.u.
This is the MO lowest in energy. It is a non-bonding orbital contributed by the 1s core atomic orbital of carbon. It contains 2 electrons (from 1s atomic orbital of carbon). As the name 'non-bonding' indicates, it does not contribute to bonding.
This MO is fully contributed by the 1s AO of carbon.
The energy is a negative value as it is a filled orbital.
MO 2
-Energy: -0.69041 a.u.
This is the MO second lowest in energy.
For construction of this orbital we will consider the combination of 1s orbitals of peripheral hydrogen atoms as ligand group orbitals (LGOs). The symmetry, shapes and phases of the LGOs and AO of central atom must be matched for construction of MOs.
It is a bonding orbital contributed by the 2s AO of carbon and in-phase 1s LGOs of hydrogens.
The MO has a greater contribution from the 2s AO of carbon than the 1s LGOs of hydrogens. This is because the 2s orbital of carbon is lower in energy than 1s orbitals of hydrogen.
It contains 2 electrons, and it has a negative energy value as a filled orbital.
MO 3
-Energy: -0.38831 a.u.
This is the highest occupied molecular orbital (HOMO). It is a bonding orbital contributed by the 2p AOs of carbon and in-phase 1s LGOs of hydrogens. There are 3 degenerate HOMOs with same energy. Each of them contains 2 electrons, and energy value is negative.
The MO has a greater contribution from the 1s LGOs of hydrogens than the 2p AOs of carbon. This is because the 1s orbitals of hydrogen is lower in energy than 2p orbitals of carbon.
The HOMOs are relatively high in energy (i.e. the energy value is close to 0), hence they are able to participate in reactions.
MO 4
-Energy: 0.11824 a.u.
This is the lowest unoccupied molecular orbital (LUMO). It is an antibonding orbital contributed by the 2s AO of carbon and out-of-phase 1s LGOs of hydrogens. It is an empty orbital and it is associated with a positive value. As an antibonding orbital, if any electron is added into it, the overall bonding will be weakened and the bond order will decrease.
The MO has a greater contribution from the 1s LGOs of hydrogens than the 2s AOs of carbon. This is because the 1s orbitals of hydrogen is higher in energy than 2s orbitals of carbon.
It is relatively low-lying (i.e. low in energy), hence it is capable of receiving electrons in reactions, resulting in breaking of C-H bonds.
MO 5
-Energy: 0.17677 a.u.
This is the first antibonding orbital higher in energy than LUMO. It is an antibonding orbital contributed by the 2p AOs of carbon and out-of-phase LGO of hydrogens. Like the degenerate HOMOs, there are 3 degenerate MOs with the same energy value as this MO. It is an empty orbital with a positive energy value.
The MO has a greater contribution from the 2p orbitals of carbon than the 1s LGOs of hydrogens. This is because the 2p orbitals of carbon is higher in energy than 1s orbitals of hydrogen.
Extra Information
Overall Bond Order = 1/2 * (number of bonding electrons - number of antibonding electrons) = 1/2 * (8-0) = 4
Bond Order for each C-H bond = 4/4 = 1
The 8 electrons are delocalised over the whole H-C-H framework, and the 4 C-H bonds are equivalent.
The MOs contain no unpaired electrons, hence, methane molecule is diamagnetic.
References
1. Appl, M. 2011. Ammonia, 3. Production Plants. Ullmann's Encyclopedia of Industrial Chemistry.
2. Appl, M. 2011. Ammonia, 3. Production Plants. Ullmann's Encyclopedia of Industrial Chemistry.
3. Peter, A. 2006. Shriver & Atkins Inorganic Chemistry. 4th ed. New Delhi: Oxford University Press.