Rep:Mod:zarfa
Module 3: Transition States and Reactivity
Introduction
The aim of this project is to analyse and characterise the transition states on a potential energy surface (PES) for the Diels-Alder cycloaddition and the Cope Re-arrangement reaction. This will be achieved by the use of computational chemistry to examine the minimum energy of transition states, reactants and products as well as enhancing our knowledge on the influence of orbital interactions on the energy of the transition states. Increased knowledge of the transition state can influence our understanding of the mechanisms by which reactions proceed, as well the observed rates of reaction amongst other properties.
The computational programes used in this project include GaussView 5.0 and Gaussian. These programes will allow us to model the PES of various molecules by calculating the total energy of the system and will determine the optimal structure of molecules involved in a reaction (using different methods). The optimised molecules can be be further investigated by carrying out frequency calculations.
The focus on this project is on two reactions:
(i) Cope Rearrangement
(ii) Diels-Alder Reaction
Cope Re-arrangement
The Cope re-arrangement reaction named after Arthur Cope, who in 1940 discovered that when a 1,5-diene is heated it undergoes a 3,3-sigmatropic rearrangment into an equivalent but more energitically stable regioisomer. An example shown below: the mechanism is an example of a pericyclic (concerted) reaction, and the transition states in this mechanism can be the chair or boat transition state.


1,5-Hexadiene: Optimisation and Frequency Analysis
The optimisation calculations will give the lowest energy minima and the transition state structures on the PES for the 1,5-hexadiene molecule. The objective of these calculations is to locate the low-energy minima and transition structures on the 1,5-hexadiene potential energy surface in order to determine the reaction mechanism by which the rearrangement proceeds.
1,5-Hexadiene has two preferred conformations it can adopt, these being the anti-peri planar and gauche conformer. The Newman projections of these structures are shown below. The Gaussian programme was used to analyse various conformers that were gauche and anti-peri planar and these were optimised to calculate the minimum energies.

In theory, there are upto 27 isomers of 1,5-hexadiene. 10 of these isomers that have different energies are outlined below. The energies are different for a number of reasons including the orbital overlap, van der Waal's interactions amongst other factors.
Why are the Gauche conformes lower in energy? This is due to the stabilisation provdided by the sigma conjugation. C-H bond donates electron density to other C-H antibonding orbitals.
The following table is taken from Appendix 1[1]
| Gauche Conformers |
|
|
|
|
|
| ||||||||||||||||||
| Energy (Hartrees) | -231.68772 | -231.69167 | -231.69267 | -231.69153 | -231.68962 | -231.68916 | ||||||||||||||||||
| Point group | C2 | C2 | C1 | C2 | C1 | C1 | ||||||||||||||||||
| Anti-peri planar Conformers |
|
|
|
| ||||||||||||||||||||
| Energy (Hartrees) | -231.69260 | -231.69254 | -231.68907 | -231.69097 | ||||||||||||||||||||
| Point group | C2 | Ci | C2h | C1 |
Anti-peri planar conformer
To carry out optimisation calculations different methods can be used. Usually, higher level basis sets (6-31G) compared to lower ones (3-21G) give energies that are similar to literature values.
A 1,5-hexadiene molecule was created on GaussView with an anti-peri planar bond. This molecule was then "Cleaned" and optimised using the following settings: Hartree-Fock method and a 3-21G basis set. Finally, the file was submitted to the HPC server to carry out the calculation.Furthermore, the point group symmetry of the molecule was Ci which indicates there is internal conversion taking place.
The D-space published link can be found here
Gauche conformer
To calculate the energy of the gauche conformer, the previous molecule was altered to create a gauche linkage. The same settings were applied as before and again the HPC server was used to carry out the calculations. The point group symmetry is C1. The D-space published link can be found here The results indicate:
Gauche energy: -231.68771613 a.u.
Anti energy: -231.69260235 a.u.
The Gauche conformer has a greater energy than the Anti-peri planar conformer. The steric hinderance of the two carbon double bond groups may be the reason for this energy difference. We will investigate the reasons for energy differences in the next section.
The Energy of the different conformers
The energy of different conformers is dictated by stereoelectronic effects. The stereoelectronic effect can be described as an electronic effect that impacts on the structure, properties and reactivity of a molecule. It can be used to relate the electron structure to the geometry of a molecule.
The table below summaries the energies of the different conformers.
The lowest energy conformer is Gauche 3 with C1 symmetry.
(i) The Van der Waals interactions that exist between the hydrogen atoms are at a maximum
(ii) The Pauli repulsion that exists between neighboring C-H bonds is reduced in this conformation
(iii) Lastly, there is the donation of the electron density from the πC=C orbital to the σ*C-H orbital resulting in stabilisation of the bond
Theory suggests the lowest energy conformer is where there is an anti-periplanar relationship between the carbon atoms. However as indicated above the data does not agree with theory.
Optimisation of the Ci anti-peri planar conformer
A 1,5-hexadiene molecule was drawn in GaussView with Ci symmetry. Initially it was optimised using the HF method and 3-21G basis set, this was sent to the HPC server to carry out the calculation. The D-space published link can be found here
After this, a higher level basis set was used: B3LYP method and 6-31G(D) basis set. The higher level basis set should give a more accurate picture of the system The calculation was then run on a HPC server. The D-space published link can be found here
The results indicate that when using the higher level basis set, it decreases the energy of the molecule which corresponds to a more stable molecule. Using the 6-31G basis set also gives a value of the energy which is closer to the literature value.[2]. Furthermore, the values of the bond lengths for the C-H and C-C bonds are also slightly different.
| Bond | 3-21G | 6-31G |
| C-C (Å) | 1.55 | 1.56 |
| C=C (Å) | 1.32 | 1.32 |
| C-H (Å) | 1.07 | 1.08 |
Frequency analysis of the Ci anti-peri planar conformer
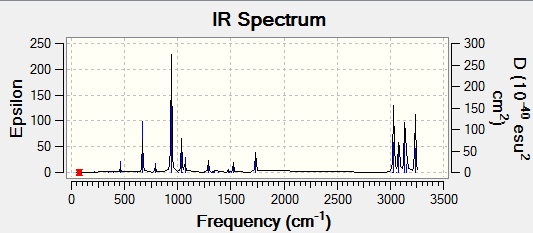
Frequency analysis is an important tool in computational chemistry. Frequency analysis is used to calculate the minimum structure on the potential energy surface and can determine the frequencies of the vibration. If the frequencies have positive values then it corresponds to the minimum however, if one or more of the values are negative it corresponds to the transition state.
Generally, for N number of atoms in a molecule there are usually 3N-6 vibrational modes. For a vibration to result in a peak, there must be a change in the dipole moment of the molecule (hence, must be IR active). Therefore, generally symmetric stretches will not appear on the spectrum. The same method was used in the frequency analysis (6-31G) on the previously optimised molecule. The D-space published link can be found here
Low frequencies --- -9.5208 -0.0005 0.0004 0.0007 2.8649 11.0477 Low frequencies --- 74.3242 80.8799 121.4080
Below the IR spectrum indicates that there are 21 vibrational peaks. However, in total there are actually 41 vibrations that occur however for peak to occur on the IR spectrum, it has to be active- there has to be a change in the dipole moment when interacting with an IR wave.
Frequency analysis also gives thermochemical data (which can be used to calculate the activation energy of the transition states).
Sum of electronic and zero-point Energies= -234.469202 Sum of electronic and thermal Energies= -234.461855 Sum of electronic and thermal Enthalpies= -234.460911 Sum of electronic and thermal Free Energies= -234.500777
| Equation and Values | ||||
| Property | Equation | Value | ||
| Sum of electronic and zero-point Energies | E(0 K) = Eelec + ZPE | -234.469202 | ||
| Sum of electronic and thermal Energies | E(298.15 K) = E(0 K) + Evib + Erot + Etrans | -234.461855 | ||
| Sum of electronic and thermal Enthalpies | H = E (298.15K) + RT | -234.460911 | ||
| Sum of electronic and thermal Free Energies | G = H - TS | -234.500777 | ||
Optimization of "Chair" and "Boat" Transition State Structures
This part of the project will focus more on the possible structures for the transition state, specefically the chair and boat structures will be optimised and analysed using various methods.
(i) Method 1: A guess transition structure is drawn and optimised using the "TS Berny" function
(ii) Method 2: This involves freezing the reaction co-ordinate and optimising the rest of the molecule, and then unfreezing and optimising the rest of the structure.
(iii) Method 3: Optimisation using the "QST2" method (does not involve a guess structure) but involves inputting the reactant and product structures to calculate the transition state structure
Method 1: Hessian Method
To create the guess transition state structure for the chair transition state, two allyl fragment (C3H5) were created and positioned in a chair transition state like structure (2.20Å distance between the terminal carbons).The results can be found on this link.
After this, the chair transition state was optimised, the job type used was 'Opt +Freq', 'TS (Berny)', the method was HF and the basis set as 3-21G, lastly the keyword chosen was 'opt=noeigen' (prevent the system from stopping if more than one imaginary frequency was detected).
here
There is a negative vibration (imaginary frequency) at -817.9780 which corresponds to the transition state of the molecule. It essentially confirms the correct formation of the chair transition state.
Low frequencies --- -817.9780 -3.5381 -1.0455 -0.0006 -0.0005 -0.0005 Low frequencies --- 1.1751 209.4810 395.9153
Zero-point correction= 0.152623 (Hartree/Particle) Thermal correction to Energy= 0.157982 Thermal correction to Enthalpy= 0.158927 Thermal correction to Gibbs Free Energy= 0.124116 Sum of electronic and zero-point Energies= -231.466700 Sum of electronic and thermal Energies= -231.461340 Sum of electronic and thermal Enthalpies= -231.460396 Sum of electronic and thermal Free Energies= -231.495206
Method 2: Frozen Co-ordinated Method
Frozen Co-ordinate method:
This method was similar to the first in that a guess chair transition state structure was created. The coordinates for the terminal atoms on the allyl fragments that form transition state were fixed to 2.20 Å. The redundant coordinates settings were changed to "Bond" and "Freeze Coordiantes". Furthermore, the structure was then optimised using the HF method and the 3-21G basis set. This avoids the complications of the Hessian method especially in relation to the force constant calculations.
There is the imaginary frequency vibration at 817.53 (it is less than the previous method) this is because the bond lengths longer and therefore weaker.
The results can be found on this link
The second part to this method was to unfreeze the co-ordinates and the change the setting to "Bond" and "Derivative", this essentially optimised the bonds that had been frozen by finding the derivative from the optimised PES. The results are shown:
here
Comparison of method 1 and 2
Bond lengths of terminal atoms (3.d.p): 2.024Å and 2.020Å
Energies: -231.61932242 and -231.61509647
Both methods give very similar data, suggesting both methods are effective in calculating the transition state structures. The advantage of method 2 is that it does not involve the hessian settings and therefore is faster than method 1, and the guess structure does not need to be drawn as accurately for method 2.
Method 3: Boat Transition State (QST2)
In order to optimise the boat transition state, two of the optimised 1,5-hexadienes (anti-peri planar,Ci) were used to represent the reactant and product respectively. The calculation type used was "Opt+Freq" with a "QST2" transition state, the method was HF and basis set set to 3-21G. However, this optimisation failed as the reactant and product geometries were not very similar to the required boat transition state, as the picture below indicates, the method QST2 did not consider the rotation around the central bonds. Thus, the structure is more representative of a chair transition state as it can not find the boat transition state. This method would only work where the reactant and product geometries are identical (or very similar).
The results can be found on this link
In order for the QST2 to work, the geometries of the product and reactant was adjusted by changing the dihedral and bond angles of the central bonds to get the structure more similar to the boat transition state structure. The central C-C-C-C dihedral angle was set to 0° and the inside C-C-C angle was set to 100°.
The results can be found on this link
Low frequencies --- -840.2338 -1.5198 -0.0002 0.0008 0.0020 4.2776 Low frequencies --- 7.9488 155.3839 382.0904
An imaginary frequency was found to occur at -840.2338cm-1 which confirms the formation of the correct boat transition state.
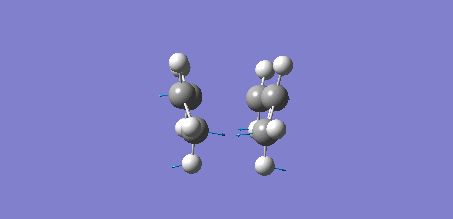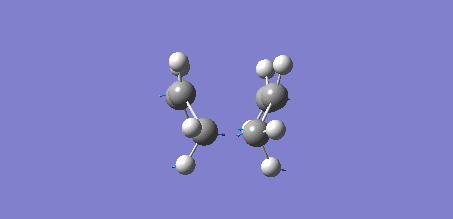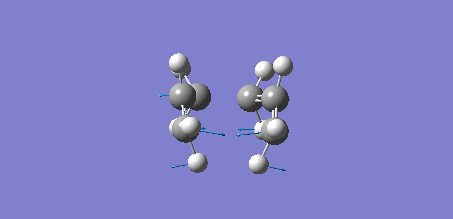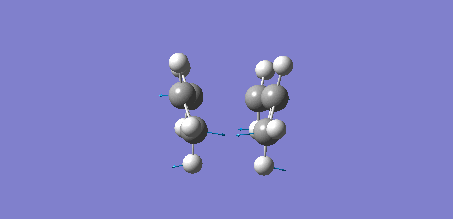
Intrinsic Reaction Coordinate (IRC) Analysis of Chair Transition State
The intrinsic reaction co-ordinate is the minimum energy pathway from the transition state to the reactants or products of a reaction "in mass wieghted cartesian on a PES. An IRC follows the path of a chemical reaction. IRC analysis calculates the local minimum point to the saddle transition state. For example, if one starts at the transition state of a chemical reaction, it can slide down the energy path to the local minimum (reactants or products). IRC analysis can give height of barrier in a reaction and information on thermodynamic properties, reaction rate, etc. This analysis works by continously changing the geometry of the molecule during the reaction. An IRC is used on the optimised chair structure to calculate the 1,5-hexadiene conformer it corresponds to.
Initally, 50 points were taken in the forward direction only. This was similar to the second gauche conformer (in Appendix 1). The results are as follows:
The results can be found on this link
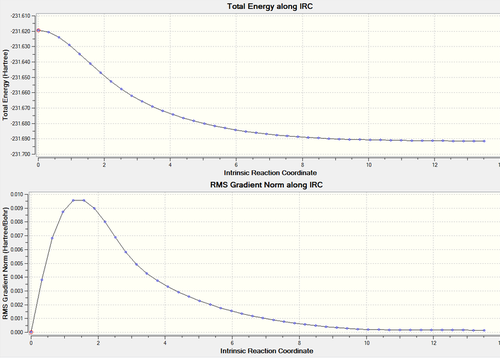
This IRC did not result in the optimised chair conformer, therefore, below a different method was tested and analysed to obtain the optimised chair conformer.
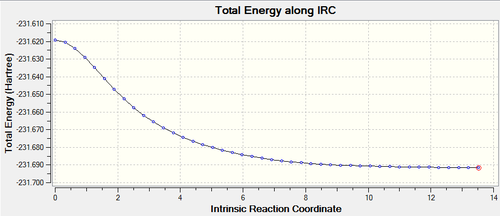
Initially, the IRC failed to reach the optimised geometry using 50 points, therefore the IRC was rerun using a larger range of points, for instance 100 points were used along the IRC. Afterwards, the lowest energy structure was optimised. This is a more effective way of carrying out IRC analysis as the more points used will result in a more accurate picture of the structure.
Lowest energy at -231.69157871 a.u.
The results can be found on this link
Calculation of Boat and Chair Activation Energies
As stated earlier, in the Cope rearrangement reaction of 1,5-hexadiene there are two pathways that go via the chair and boat transition state structures. It is possible to calculate the activation energies for these respective pathways by analysing the structures using a higher basis set of 6-31G(D), as well as carrying out frequency analysis.
Chair Transition State
The results can be found on this link
Item Value Threshold Converged?
Maximum Force 0.000058 0.000450 YES
RMS Force 0.000019 0.000300 YES
Maximum Displacement 0.000611 0.001800 YES
RMS Displacement 0.000155 0.001200 YES
Predicted change in Energy=-1.196705D-07
Optimization completed.
-- Stationary point found.
Frequency Analysis of the Chair Transition State
The results can be found on this link
Zero-point correction= 0.142051 (Hartree/Particle) Thermal correction to Energy= 0.147973 Thermal correction to Enthalpy= 0.148917 Thermal correction to Gibbs Free Energy= 0.113165 Sum of electronic and zero-point Energies= -234.414932 Sum of electronic and thermal Energies= -234.409010 Sum of electronic and thermal Enthalpies= -234.408066 Sum of electronic and thermal Free Energies= -234.443818
In the second line, the values are positive, these refer to the imaginary frequency and correspond to the presence of the optimimum structure.
Low frequencies --- -565.6905 -0.0004 0.0007 0.0010 22.0392 27.0711 Low frequencies --- 40.1427 194.4985 267.7693
Boat Transition State
The results can be found on this link
Item Value Threshold Converged?
Maximum Force 0.000009 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000128 0.001800 YES
RMS Displacement 0.000052 0.001200 YES
Predicted change in Energy=-2.676308D-09
Optimization completed.
-- Stationary point found.
Frequency analysis of the boat transition state structure. The results can be found on this link
In the second line, the values are positive, these refer to the imaginary frequency and correspond to the presence of the optimimum structure.
Low frequencies --- -530.3618 -8.3908 -0.0006 -0.0004 -0.0003 15.4632 Low frequencies --- 17.6137 135.6121 261.7006
The lowest "real" normal mode is found to occur at 135.6121cm-1
Zero-point correction= 0.140751 (Hartree/Particle) Thermal correction to Energy= 0.147086 Thermal correction to Enthalpy= 0.148030 Thermal correction to Gibbs Free Energy= 0.111341 Sum of electronic and zero-point Energies= -234.402342 Sum of electronic and thermal Energies= -234.396008 Sum of electronic and thermal Enthalpies= -234.395063 Sum of electronic and thermal Free Energies= -234.431752
Activation Energies Summary
The energies of the transition states are:
Chair: -234.55698262 a.u.
Boat: -234.54309307 a.u.
The activation energy at 298 K can be determined by calculating the difference between the sums of the electronic and thermal energies of the transition states and product. Furthermore, activation energy at 0 K can be determined by calculating the difference between the sums of the electronic and zero-point energies of the transition state and reactant.
| Transition State | Activation Energy (6-31G) at 0 K (2 dp)(KCal/mole) | Activation Energy (6-31G) at 298 K (2 dp) (KCal/mole) | Activation Energy (3-21G) at 0 K (2 dp)(KCal/mole) | Activation Energy (3-21G) at 298 K (2 dp) (KCal/mole) | Literature Values |
|---|---|---|---|---|---|
| Chair | 34.36 | 33.16 | 45.72 | 45.30 | 33.5 ± 0.5 |
| Boat | 41.63 | 41.07 | 55.61 | 50.32 | 44.7 ± 2.0 |
Overall, using a higher level basis set during the analysis of the transition states gives us a more accurate picture of the system and especially its energy. The results indicate that the favoured pathway may be the one in which there is a chair transition state due to the lower activation energy.
The reaction may go via a boat transition state structure if the conditions are favourable (thermodynamic conditions) as the system requires thermal energy to mount the barrier. Under kinetic conditions (low temperature) the chair transition state is favoured as it requires less energy to overcome the barrier[3].
At increased temperatures (289K) the activation energy decreases as the kinetic energy of the molecules increases, compared to lower temperatures (0K).
Finally, the sum of the electronic and zero-point energies of the molecules are lower than the sum of the electronic and thermal energies, this is becuse at 0K there are no thermal vibrations.
The Diels-Alder Cycloaddition Reaction
A Diels-Alder cycloaddition reaction can be described as a "concerted" [4+2] cycloaddition. The reaction involves an alkene dienophile with a conjugated diene, there are also 4n+2 pi electrons which results in a stable cyclic alkene. The Diels-Alder is an interesting and useful reaction due to its stereo-selective and regio-selective properties. The rate of the reaction can be influenced by EWG (increase) on the dienophile (lowers the energy of the pi* orbital so increases the overlap).
A Diels-Alder reaction involves the interaction of the HOMO of one species with the LUMO of another. The two Diels-Alder reactions will be investigated using GaussView and Gaussian in order to determine and analyse the transition state structures and determine the HOMO and LUMO orbitals of the species.
The two Diels-Alder reactions:
(1) ethylene (dienophile) with cis-butadiene (diene)
(2) maleic anhydride (dienophile) with cyclohexa-1,3-diene (diene)
Ethene with Cis-Butadiene
When the ethylene reacts with the cis-butadiene it results in the formation of two sigma bonds between the molecules. The mechanism is shown below:
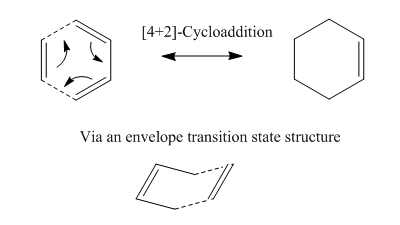
Reactivity is controlled by relative energies of Frontier Molecular Orbitals. The butadiene must be in the cis conformation to enable efficient overlap. The molecular orbitals of the reactants and transition states were determined using Gaussian.
Optimisation of the molecules (butadiene and ethene)
It is important to consider why the reactants form the specific (envelope) transition state structure. Therefore, the molecular orbitals need to be analysed for both ethene and cis-butadiene. These reactants were optimised using a method that was semi-empirical and with a AM1 approach. The results are as following:
Cis-butadiene
The results can be found on this link
Ethene
The results can be found on this link
The optimisation for both reactants was successful, as indicated by the results RMS gradient values - corresponding to the minimum point on the potential energy surface where the gradient is zero.
Molecular Orbitals
Furthermore, the molecular orbitals were determined using the formatted checkpoint file (FCHK) and are shown below:
Cis-butadiene: the HOMO orbital has a node between carbon atom 2 and carbon atom 3. The LUMO has two nodes and is an anti-bonding orbital.
Ethene: the HOMO is a completely bonding orbital (no nodes are present), there is overlap of two p orbitals. The LUMO has one node (anti-bonding orbital).
Diagram shows the frontier orbital for the cis-butadiene and for the ethene molecule.
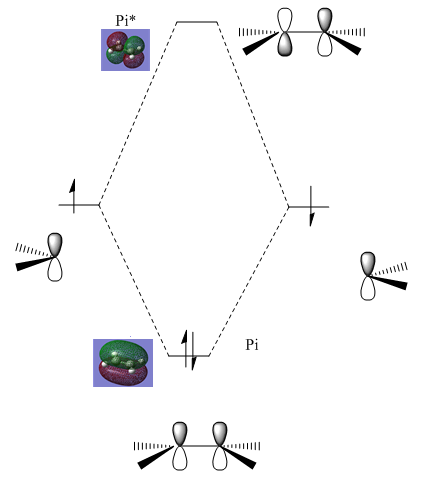
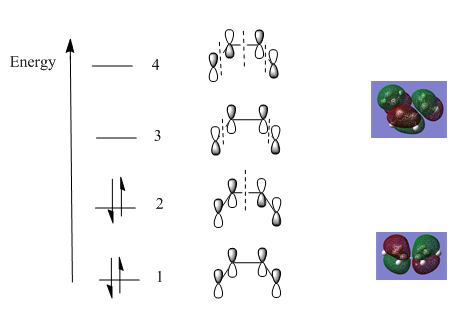
Which orbitals overlap?
For this question, we must consider the symmetry and energy of the frontier orbitals for each molecule. For orbitals to overlap efficiently it is required they have the same symmetry and are similar in terms of energy. Therefore, the HOMO of the cis-butadiene may overlap with the LUMO of the ethene molecule as both are asymmetric. And the HOMO of the ethene may overlap with the LUMO of the cis-butadiene.
Relative energy differences:
(i) 0.40484 a.u.
(ii) 0.39665 a.u.
The value of (i) is greater than(ii) therefore indicating there is stronger overlap of the asymmetric orbitals. This will contribute towards the transition state structure. But energy differences are very close so needs to be investigated further. The MO diagram above indicate that the "real" molecular orbitals are not different from the molecular orbitals formed from the linear combination of atomic orbitals, as a result validating the qualitative MO theory. It proves it can be an accurate tool to model the molecular frame work of simple molecules. MO theory tends to break down when the molecules get more complex and therefore it eventually requires a quantum mechanical description.
Calculation of Optimal Transition State Structures via Frozen Coordinate Method
In this method, the transition state (which is supposed to be an envelope structure), is drawn on GaussView based on a bicycle-2,2,2-octane fragment. The structure was optimised using the semi-empirical AM1 method and using the frozen co-ordinate method. Where the terminal carbons of the transition state were set to 2.20A.
Optimisation
The results can be found on this step 1 link and this step 2 link
Frequency analysis
Frequency analysis was also carried out using the same method (semi-empirical AM1). The values indicate the presence of a large imaginary frequency at -935.5938, which corresponds to the formation of the sigma bond and loss of the pi character in the system.
The results can be found on this link
Zero-point correction= 0.141783 (Hartree/Particle) Thermal correction to Energy= 0.148103 Thermal correction to Enthalpy= 0.149048 Thermal correction to Gibbs Free Energy= 0.112360 Sum of electronic and zero-point Energies= 0.263447 Sum of electronic and thermal Energies= 0.269767 Sum of electronic and thermal Enthalpies= 0.270711 Sum of electronic and thermal Free Energies= 0.234024
Low frequencies --- -935.5938 -137.6503 -78.3279 -68.1294 -0.0057 -0.0015 Low frequencies --- 0.0070 142.8641 246.0153
The vibration corresponds to the (pericyclic reaction) formation of the sigmabond and loss of the pi-character. During the reaction, there is a change in the hybdrisation of the ethene molecule from sp2 to sp3.
The vibration shows how the ethene molecule interacts with the butadiene and gives an indication of how the HOMO/LUMO orbitals align and interact to form the bonds.
The terminal bond length of the transition state is greater than the literature values, indicating that the carbon to carbon bond has not formed. But the length is small enough to suggest that orbital interaction is taking place as the distance is smaller than the van der waals radius of a C atom.
MO Analysis of Envelope Transition State
The molecular orbitals of the envelope transition state are:
Reaction: Cyclohexadiene and Maleic Anhydride
The second Diels-Alder cycloaddition reaction under investigation is the one between maleic anhydride and cyclohexadiene. The product can be either an exo and/or endo. The exo product is more abundant under thermodynamic conditions, whereas under kinetic conditions the endo product is favoured[4]. This can be explained by considering the influence of orbital interactions in the transition state, and will be under investigation using Gaussian.
Optimisation of the molecules
Maleic anhydride
The results can be found on this link
Cyclohexadiene
The results can be found on this link
Molecular Orbital Analysis
The energy gap between the HOMO and LUMO orbitals in maleic anhydride is greater than the one in cyclohexadiene. Maleic anhydride would be able to accept electrons from the HOMO of the cyclohexadiene due to this favourable energy and also because both orbitals are antisymmetric.
The HOMO of the cyclohexadiene molecule has weakly bonding character, it is symmetrical and has a node in the orbital located centrally. The LUMO has a greater number of nodes and therefore can be considered to have greater antibonding character.
The HOMO of the maleic anhydride appears to have strong bonding character, this includes the bonding interactions between the ring and the oxygens. The LUMO has greater antibonding character, which is also suggested by the higher number of nodes on the carbonyl and alkene bonds.
Analysis of Endo and Exo Transition States
The previously optimised molecules were used to create an exo transition state structure. This exo structure was then optimised using the forzen coordinate method using the DFT/6-31(G) level. The terminal carbons were frozen at 2.10 Å.
Exo Transition State
Optimisation The results can be found on this link
Length of bond being formed: 2.17Å
Frequency Analysis
The results can be found on this link
(Hartree/Particle) Thermal correction to Energy= 0.190639 Thermal correction to Enthalpy= 0.191583 Thermal correction to Gibbs Free Energy= 0.145270 Sum of electronic and zero-point Energies= -612.479719 Sum of electronic and thermal Energies= -612.469915 Sum of electronic and thermal Enthalpies= -612.468971 Sum of electronic and thermal Free Energies= -612.515284
Low frequencies --- -469.6135 -71.0506 -46.0291 -18.2869 -0.0011 -0.0008 Low frequencies --- -0.0007 28.8459 58.1235
Endo Transition State
Optimisation
The results can be found on this link
Length of bond being formed: 2.16Å
Frequency Analysis The results can be found on this link
Thermal correction to Energy= 0.191764 Thermal correction to Enthalpy= 0.192708 Thermal correction to Gibbs Free Energy= 0.144448 Sum of electronic and zero-point Energies= -612.496507 Sum of electronic and thermal Energies= -612.485959 Sum of electronic and thermal Enthalpies= -612.485014 Sum of electronic and thermal Free Energies= -612.533275
Low frequencies --- -476.2820 -46.1649 -26.6727 -13.5480 -0.0011 -0.0009 Low frequencies --- -0.0005 46.8498 103.3937
Discussion of Endo and Exo Transition State Structures
It is important to consider the reasons as to why the exo product is favoured under thermodynamic conditions and the endo product under kinetic conditions. Using Guassian and GaussView it enables us to analyse the optimised structures and calculate the activation energies to suggest reasons why the products may be favoured under certain conditions.
| Parameter | Exo | Endo |
|---|---|---|
| Inter-fragment Bond Length (C-C) | 2.29 | 2.27 |
| Bond Length in Maleic (C-C) | 1.48 | 1.48 |
| Average Bond Length in cyclohexadiene (C-C) | 1.53 | 1.54 |
| Bond Length sp2 to sp3 (C-C) | 1.40 | 1.39 |
| Bond Length Former sp3 to sp2 (C=C) | 1.40 | 1.40 |
| Through Space Distance (C=C)-(C-C) | 2.96 | 2.87 |
The table above displays information on the exo and endo structures, including the bond lengths. It can be seen that both products are very similar. However, there is a small difference in the geometry of the products, especially in the inter-fragment bond lengths (0.02 Å).
Bond Lengths
To explain the differences we also must consider the overall mechanism. The process involves two electrons going from pi bonds to sigma bonds, a new pi bond is also formed on the diene. On the maleic anhydride the C=C converts to a C-C bond (pi to sigma). The double bond that forms in the structures is the same bond length (1.40) in the exo and endo transition state. The C-C bond that forms from the previously C=C bond is also 1.40 in both the endo and exo transition states. Both of these values are different to the literature values, where the C=C is 1.34 A and the C-C is 1.54. More importantly, the product bond lengths are not the same and therefore we cannot define the exo or endo structures as early or late transition states.
Through Space Distances
Although both structures have steric hinderance as both through space distances are smaller than the C-C Van der Waals distance of 3.2 Å[5]. The through space distance is greatest for the exo structure at 2.96 Å compared to the 2.87 Å for the endo transition state structure. Therefore, this suggests there is less steric repulsion between the two molecules in the exo structure. As a result, the exo product is most thermodynamically favoured as they have less repulsive energy[6].
The endo product is most common in Diels-Alder reactions, suggesting the activation energy for the endo pathway is lower than the exo. The values for the activation energies are given in the table below:
| Thermodynamic Value | Exo | Endo |
|---|---|---|
| Total 'Sum of electronic and thermal energies' for the fragments (maleic anhydride + cyclohexadiene)(au) | -612.53459 | -612.53459 |
| Sum of electronic and thermal energies for the transition states (au) | -612.481458 | -612.488646 |
| Activation Energy (au)) | 0.053 | 0.046 |
| Activation Energy (kCal mol-1) (3 dp) | 33.40 | 28.77 |
As suggested the activation barrier is smaller for the endo transition state structure than the exo transition state structure. As a result for kinetic conditions, when there is no thermal energy to overcome the exo barrier the endo product will be favoured as it forms the fastest, and there is not enough energy in the system to overcome the activation barrier to go back to the reactants. The greater stability of the endo structure is because of the secondary orbital overlap in the frontier orbitals. Secondary orbital overlap is shown below:
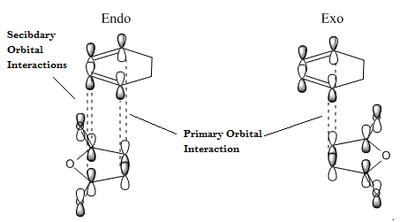

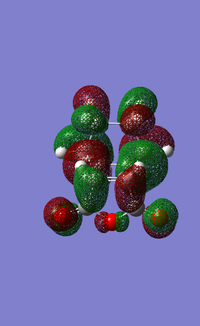
The HOMO of the cyclohexadiene interacts with the LUMO of the maleic anhydride, this makes sense as both orbitals are antisymmetric.
Overall, the exo product has a lower energy than the corresponding endo product due to stabilising interactions in the exo product. However, the endo product pathway has a lower activation energy barrier than the exo.
The HOMO for the endo product has a higher energy because of the repulsive interaction between the π*C=O, these interact with the π*C=C. The exo species has no such repulsive interactions and is the more thermodynamically favoured product.
Conclusion
This project has demonstrated the advantage of using computational chemistry to study transition state structures. It has been shown that using programmes such as Gaussian and GaussView not only can optimise molecules but also allow chemists to study the kinetic and thermodynamic observations in reactions.
Transition state structures are very difficuilt to study using classic scientific techniques. Therefore, by using computational methods it allows chemists to gather optimisation and frequency data from these transition state structures and explain any properties.
In the first part of the project, the Cope Re-arrangement reaction was analysed and it was seen that the reaction proceeded via a chair transition state structure.
In the second part of the project two Diels-Alder reactions were analysed. Firstly, the reaction between ethene and cis-butadiene, this was found to proceed via an enveloped transition state and the reaction was influenced by the frontier molecular orbital interactions. The second reaction was the one between cyclohexadiene and maleic anhydride and it was found that the endo product was favoured over the exo product (in kinetic conditions). This again was due to the orbital interactions that existed.
References
- ↑ https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:phys3.
- ↑ G. Schultz, I. Hrgittai, J. Mol. Struct., 1995, 346,63-69
- ↑ Keiji. Morokuma , Weston Thatcher. Borden , David A. Hrovat, J. Am. Chem. Soc., 1988, 110 (13), pp 4474–4475
- ↑ James H. Cooley and Richard Vaughan Williams; J. Chem. Educ., 1997, 74 (5), p 582
- ↑ J. Bondi, A. (1964). "Van der Waals Volumes and Radii". J. Phys. Chem. 68 (3): 441–51.
- ↑ Clayden, J.; Greeves, N.; Warren, S.; Wothers, P.; Organic Chemistry, 2011, Oxford University Press