Rep:Mod:yukiching
Conformational analysis
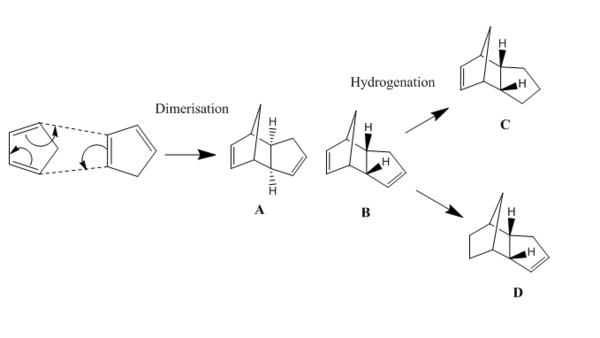
The Hydrogenation of Cyclopentadiene Dimer
Introduction
Dimers A and B are formed from the dimerisation of two cylopentadiene. Dimer B, the endo dimer specially undergo hydrogenation for a long term, forming dimer C and D.The aim of the project is to use the molecular mechanics technique to determine dimers B and C is whether kinetically or thermodynamically controlled. The molecules are firstly build up via ChemDraw Pro 13.0, then optimized and analysed by Avogadro.
Analysis and Discussion
The table below provides several data for the four molecules.(MMFF94s)
| Property | A | B | C | D |
| Bond Stretch | 3.543 | 3.467 | 3.308 | 2.823 |
| Angle Bend | 30.773 | 33.191 | 30.865 | 24.685 |
| Stretch-bending | -2.041 | -2.082 | -1.927 | -1.657 |
| Torsion | -2.731 | -2.950 | 0.059 | -0.378 |
| VDW | 12.801 | 12.358 | 13.282 | 10.637 |
| Out-of-plane Bending | 0.015 | 0.022 | 0.015 | 0.000 |
| Electrostatic Interaction | 13.014 | 14.185 | 5.121 | 10.637 |
| Total energy (kcal/mol) | 55.373 | 58.191 | 50.723 | 41.257 |
Optimized geometry of each dimers
| A | B | C | D | ||||||||||||
|
|
|
| ||||||||||||
From the above table, it is clear that the total energy of dimer A is lower than that of B, which means that dimer A (exo) is more thermodynamically stable than dimer B (endo). Comparing the data for A and B, angle bending energy contribute the mainly difference. The angle shown on the geometry also obtain that structure of A is more bending than B. According to the fact that dimer B is the major product of dimerization, we can occur that this reaction is kinetic control.
For the data of dimer C and D, we can obtain that dimer D is more thermodynamically stable than C, since the total energy of C is much greater than D. This is mostly due to the large differences of angle bending, van der waals forces and electrostatic interaction. Assuming that the reaction is under kinetic control, dimer C with higher energy will form much faster than dimer D. However, dimer C will slowly convert into dimer D in order to minimize the total energy.
Introduction
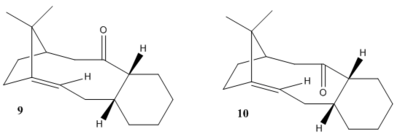
Taxol is synthesised from the carbonly group pointing either up or down. Compound 9 and 10 shown below are the key intermediates of Taxol's synthesis. Atropisomerism is formed due to restricted rotation about single bond where the high steric demand involves. The stereochemistry of canbonly addtion depends on which isomer is the most stable. The objective of this part is to determine which intermediates is more stable.
Analysis and Discussion
Table 1
| 9&10 | 9 | 10 | ||||||
 |
|
| ||||||
Table 2
| 9&10 | 9 Product | 10 Product | ||||||
 |
|
| ||||||
Table 1 is the data and geometry of compound 9 and 10. Comparing the total energy, it shows that compound 10 is more thermodynamically stable than 9.
Table 2 is the data and geometry of the final products from intermediates 9 and 10.Hence, the OS value of intermediate and its product can be obtain (The torsion energy difference between the intermediate and its product), which is -10.403 kcal/mol and -9.340 kcal/mol for 9 and 10 respectively.As the OS value smaller than 17kcal/mol, the compound is defined as isolable bridgehead olefins.[1] As a result, functionalisation of the alkene react much slowly.
Spectroscopic Simulation using Quantum Mechanics
Introduction

In this section, the structure of compound 17 is build up by Chem Draw and optimized by Avogadro.Then the molecule data is calculated by HPC system and the 1H and 13C NMR of the model is analysed via Gaussian. The result is compared with the literature values.
Analysis and Discussion
ah_test |
| Property | 17 |
| Bond Stretch | 15.905 |
| Angle Bend | 31.575 |
| Stretch-bending | 0.226 |
| Torsion | 11.044 |
| VDW | 52.000 |
| Out-of-plane Bending | 1.239 |
| Total electrostatic energy | -7.205 |
| Total energy (kcal/mol) | 104.783 |
13C NMR
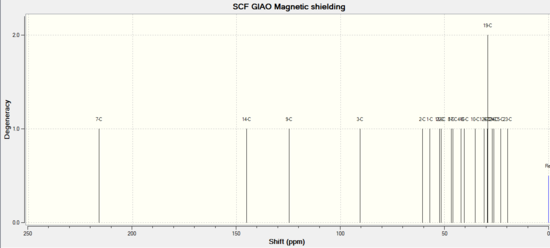
| Literature (ppm) in CDCI | Chemical shift/ppm in CHCl3 | Degeneracy | Atom |
| 218.79 | 216.11 | 1 | 7 |
| 144.63 | 145.12 | 1 | 14 |
| 125.33 | 124.68 | 1 | 9 |
| 72.88 | 90.65 | 1 | 3 |
| 56.19 | 60.64 | 1 | 2 |
| 52.52 | 57.04 | 1 | 1 |
| 48.50 | 52.48 | 1 | 11 |
| 46.80 | 51.55 | 1 | 22 |
| 45.76 | 46.69 | 1 | 8 |
| 39.80 | 45.95 | 1 | 17 |
| 38.81 | 42.17 | 1 | 4 |
| 35.85 | 40.57 | 1 | 16 |
| 32.66 | 35.33 | 1 | 10 |
| 28.79 | 31.01 | 1 | 12 |
| 28.29 | 29.34 | 2 | 6,19 |
| 26.88 | 27.09 | 1 | 13 |
| 25.66 | 26.40 | 1 | 24 |
| 23.86 | 22.90 | 1 | 5 |
| 20.96 | 19.73 | 1 | 23 |
The contribution of pin-orbit coupling errors of the carbons connecting to heavy atoms( sulphur)is significant to the value of chemical shift.It may also due to the difference solvent of each NMRs, different conformations and chemical shift corrections. Hence,the differences between t the predicted value and the literature are acceptable.
1H NMR
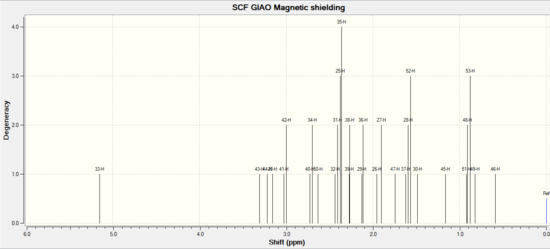
| Literature (ppm) in CDCI | chemical shift/ppm in CHCl3 | Degeneracy | atom |
| 4.84 (dd,1H) | 5.149 | 1 | 33 |
| 3.40-3.10 (m, 4H) | 3.306 | 1 | 43 |
| 3.220 | 1 | 44 | |
| 3.157 | 1 | 20 | |
| 2.99 (dd, 1H) | 3.013 | 2 | 41,42 |
| 2.80-1.35 (series of m, 14H) | 2.714 | 2 | 40,34 |
| 2.633 | 1 | 50 | |
| 2.395 | 4 | 32,31,25,35 | |
| 2.272 | 2 | 39,38 | |
| 2.120 | 2 | 29,36 | |
| 1.928 | 2 | 26,27 | |
| 1.744 | 1 | 47 | |
| 1.38 (s, 3H) | 1.594 | 3 | 37,28,52 |
| 1.25 (s, 3H) | 1.488 | 1 | 30 |
| 1.163 | 1 | 45 | |
| 1.10 (s, 3H) | 0.906 | 3 | 51,48,53 |
| 1.00-0.80 (m, 1H) | 0.823 | 1 | 49 |
| 0.590 | 1 | 46 | |
Comparing the predicted chemical shift with the literature value, the differences is significant small;considering that the solvent for each NMR are different. This may also due to different conformations and chemical shift corrections.
Reference
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem., 2013, DOI:10.1021/ja00398a003
Analysis of the properties of the synthesised alkene epoxides
The two catalytic systems
Introduction
Compound 21 and 23 is the stable pre-catalysis of Shi's Catalysis(22) and Jabcobsen's Catalysis(24).The crystal structure of Shi and Jacobsen catalyst were builf up by CCDC. We are mainly focus on the difference between two anomeric centers.
| Shi's Catalysis | Jacobsen's Catalysis | ||||||
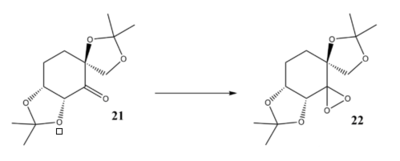 |
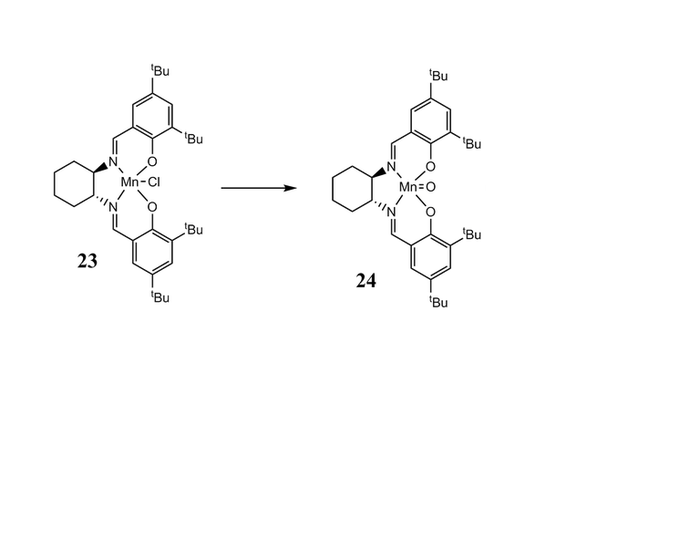 | ||||||
|
| ||||||
Discussion
Special Bond distance
| Shi's Catalysis | Jacobsen's Catalysis |
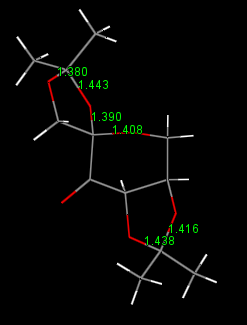 |
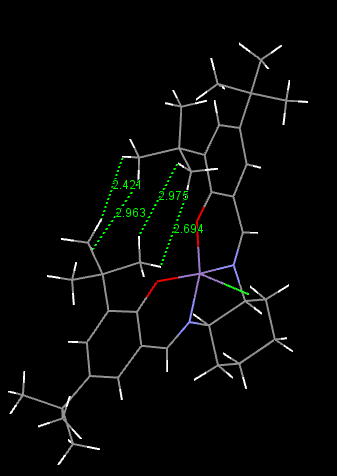 |
For the Shi's Catalysis, we are mainly focus on the bond length between anomeric centers(i.e. those with O-C-O structures).Comparing with the normarl C-O bond (143pm), the average C-O bond length in Shi catalysis is slightly shorter (141pm). This is due to the anomeric effect. When the central carbon is orthogonal to the two adjacent oxygen, the lone pair on one of the oxygen is free to donate into the empty C-O σ* anti-bonding orbital. Hence, one of the C-O is strengthen while the other is weakened since the dditional electron density on anti-bonding orbital. However, a slightly decrease in bond length is obtain.
For the Jacobsen's Catalysis, the distances between the two hydrogen on each butyl group are measured. The average value is close to the the van der Waals distance for two hydrogen ( 0.240nm)[1]. The attraction of these two hydrogen stop the alkane from approaching the metal center. Stereospecific epoxide is obtain.
Analysis NMR properties of epoxdiation products
Introduction

ah_test |
ah_test |
Discussion
Styrene Epoxide
C-NMR
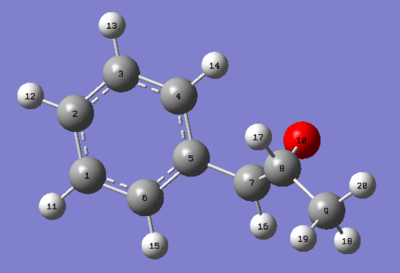
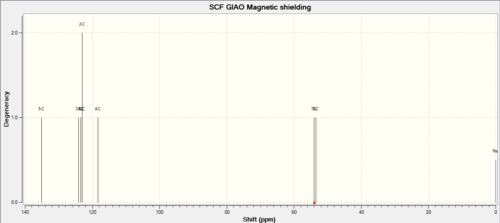
| C-NMR | Shift (ppm) | Degeneracy | Atoms |
| 135.142 | 1 | 5 | |
| 124.135 | 1 | 3 | |
| 123.414 | 1 | 1 | |
| 122.953 | 2 | 6,2 | |
| 118.268 | 1 | 4 | |
| 54.060 | 1 | 7 | |
| 53.453 | 1 | 8 |
H-NMR
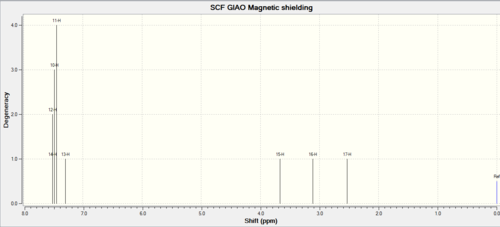
| Shift (ppm) | Degeneracy | Atoms |
| 7.489 | 4 | 14,12,10,11 |
| 7.298 | 1 | 13 |
| 3.664 | 1 | 15 |
| 3.114 | 1 | 16 |
| 2.533 | 1 | 17 |
Trans-β-Methylstyrene Epoxide
C-NMR

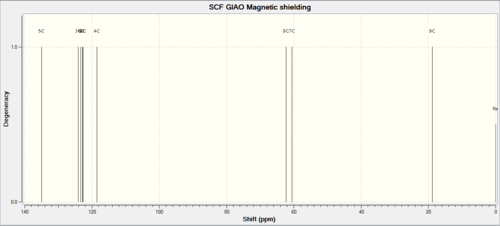
| Shift (ppm) | Degeneracy | Atoms |
| 134.981 | 1 | 5 |
| 124.073 | 1 | 3 |
| 123.328 | 1 | 1 |
| 122.794 | 1 | 6 |
| 122.727 | 1 | 2 |
| 118.488 | 1 | 4 |
| 62.306 | 1 | 8 |
| 60.586 | 1 | 7 |
| 18.833 | 1 | 9 |
H-NMR

| Shift (ppm) | Degeneracy | Atoms |
| 7.491 | 3 | 15,13,11 |
| 7.422 | 1 | 12 |
| 7.307 | 1 | 14 |
| 3.416 | 1 | 16 |
| 2.786 | 1 | 17 |
| 1.679 | 1 | 20 |
| 1.587 | 1 | 19 |
| 0.717 | 1 | 18 |
Assigning the absolute configuration of the product
Introduction
For the two epoxide below, we use the Cambridge variation on the B3LYP density functional method to calculate the optical rotation in order to determine chiroptical properties.The wavelength of the incident light is 589nm and reading [ALPHA](5890.0A) gives the estimated optical rotation for the built enantiomer. Then the result is compared with the literature values.
Discussion
Comperison of Optical Rotation[2] [3]
| ' | R,R-Styrene | Literature | S,S-Styrene | Literature | R,R-trans-β-Methylstyrene1 | Literature | S,S-trans-β-Methylstyrene1 | Literature |
| Optical Rotation Power | -172.84 deg | -24 deg | 165.34deg | 20 deg | 36.60 deg. | 40.8 deg | 40.87 | -47.8 deg |
Comparing with the literature values, it may be cause by the difference in temperature, solvent and enantiometric excess.
Properties of transition state for the β-methyl styrene oxide The value of K is calculate via the equation:G = -RTlnk
| Energy | SS | RR | difference(hartree) | J/MOL | K |
| G TS1 | -1343.018 | -1343.023 | |||
| G TS2 | -1343.016 | -1343.019 | |||
| G TS3 | -1343.024 | -1343.029 | |||
| G TS4 | -1343.025 | -1343.032 | |||
| average | -1343.021 | -1343.026 | 0.005 | 14.306 | |
| G TS4 | -1343.025 | -1343.032 | 0.008 | 20219.000 | 3501.000 |
From the above table, free energies of RR transition states are slightly larger than SS,which means that RR transition states are more energetically stable. The value of K is also agree with the statement that the epoxidation reaction will exclusively give (R,R)-trans-beta-methyl styrene oxide over than (S,S)-trans-beta-methyl styrene oxide
DCE Analysis
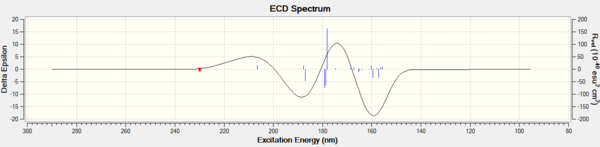
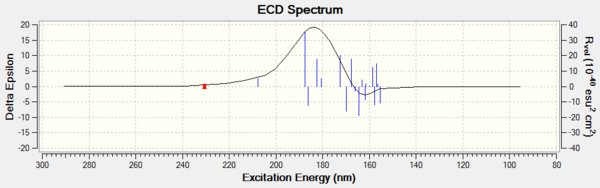
Investigating the non-covalent interactions (NCI) in the active-site of the reaction transition state
Non-covalent interactions is use to analysis the properties of electron density.The colors indicates the interaction:blue = attractive, green = mildly attractive, yellow = mildly repulsive and red = strongly repulsive.The interactions enables characterisation and identification of both stablization (hydrogen bonding in blue and dispersion in green) and destablization (steric repulsion in red).[4]
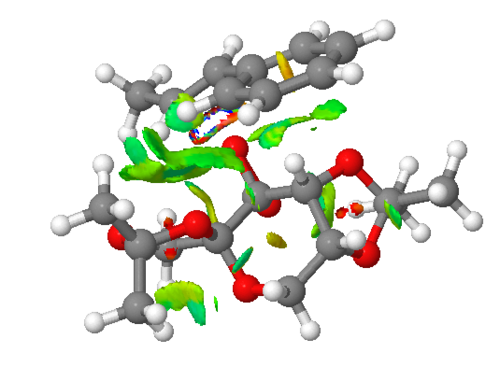
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
QTAIM is complementary to the NCI (non-covalent) analysis.Since NCI mainly analysis the weak interaction within molecule while QTATM are more focus on the electron density (and its curvature) in the covalent regions of molecules.
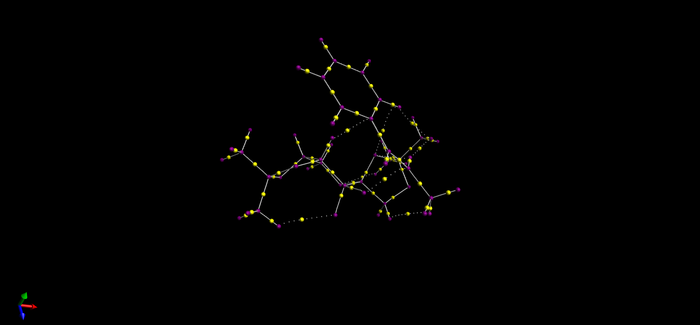
Suggesting new candidates for investigations
Search Reaxys for epoxide as substructure with the measured property ORP=500. The below compound is obtainED.
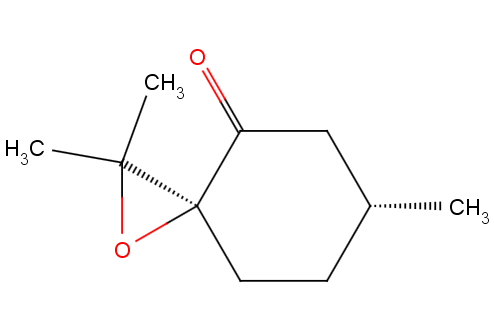
References
- ↑ Manjeera Mantina , Adam C. Chamberlin , Rosendo Valero , Christopher J. Cramer and Donald G. Truhlar *, 2009, DOI:10.1021/jp8111556
- ↑ DOI:10.1021/ja00853a029
- ↑ DOI:10.1039/B606881B
- ↑ Dr. Jannine L. Arbour1, Prof. Henry S. Rzepa1, Dr. Julia Contreras-García2,*, Dr. Luis A. Adrio1, Dr. Elena M. Barreiro1, Prof. King Kuok (Mimi) Hii1, 2012DOI:10.1002/chem.201200547
