Rep:Mod:yq5118
NH3 molecule
Information of NH3
| Name | NH3 |
| Calculation type | FREQ |
| Calculation title | RB3LYP |
| Basis set | 6-31G(d,p) |
| E9RB3LYP) | -56.5577687 a.u. |
| RMS gradient | 0.00000485 a.u. |
| Point group | C3V |
optimisation of NH3
| Bond length | 1.07198 |
| Bond angle | 105.741 |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986357D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Jmol Dynamic Range
test molecule |
You can find the optimised in the link:Media:Yq5118_NH3_OPTIMISATION.LOG
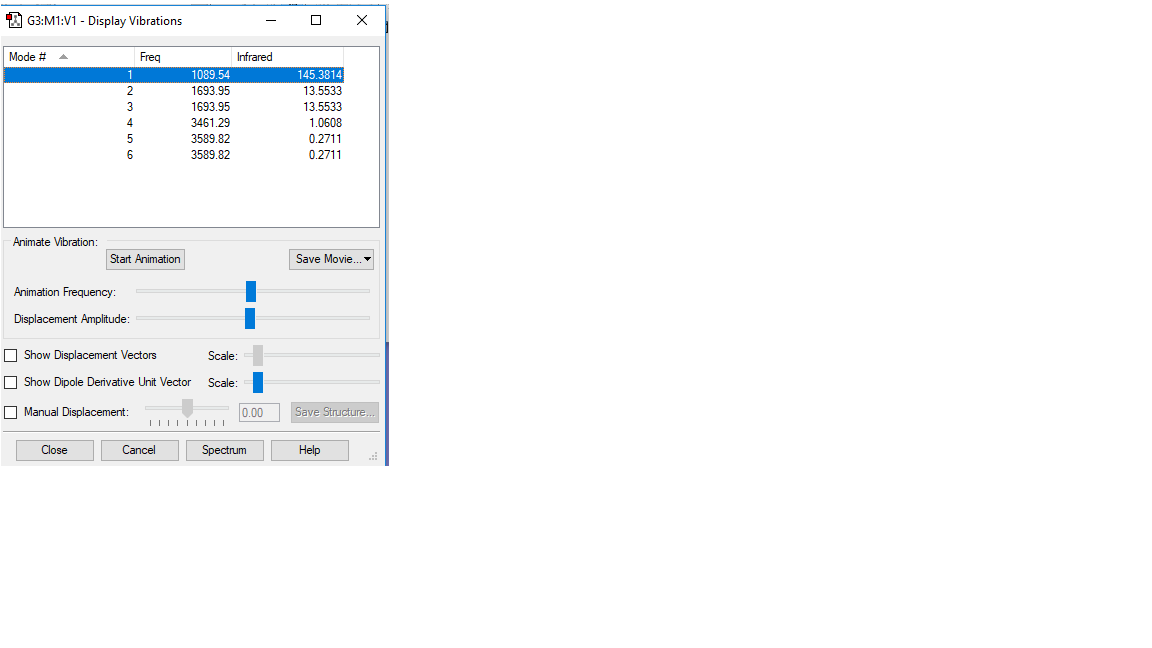
Vibration of NH3
basic information of NH3 vibration
some other information of NH3 vibration
According to 3N-6 rule, there should be 6 vibration modes.
Mode2 and mode3;mode5 and mode6 have the same energies, thus they are degenerate states.
Modes 1,2,3 are "bending" vibrations and modes 4,5,6 are "bond stretch" vibrations.
Modes 1 and 4 are highly symmetric.
Mode1 is known as the "umbrella" mode.
Four bands are expect to be seen in an experimental spectrum of gaseous ammonia
vibration mode
| Wavenumber
cm-1 |
1090 | 1694 | |
| Symmetry | A1 | E | |
| Intensity
arbitrary units |
145.4 | 13.55 | |
| Image | 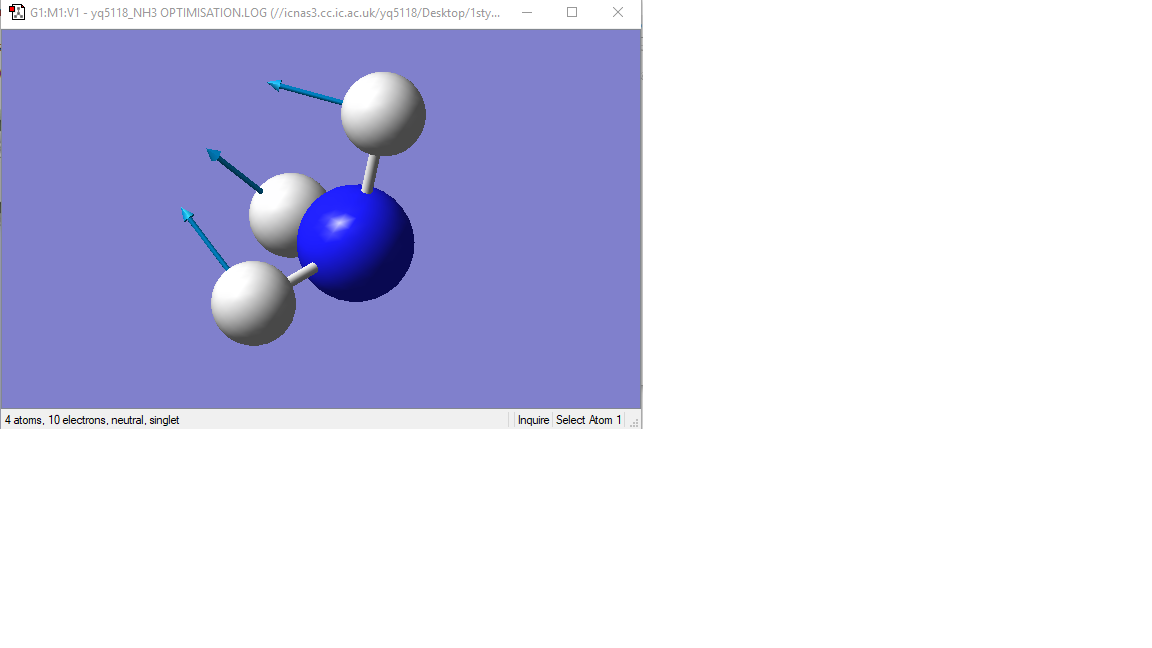
|
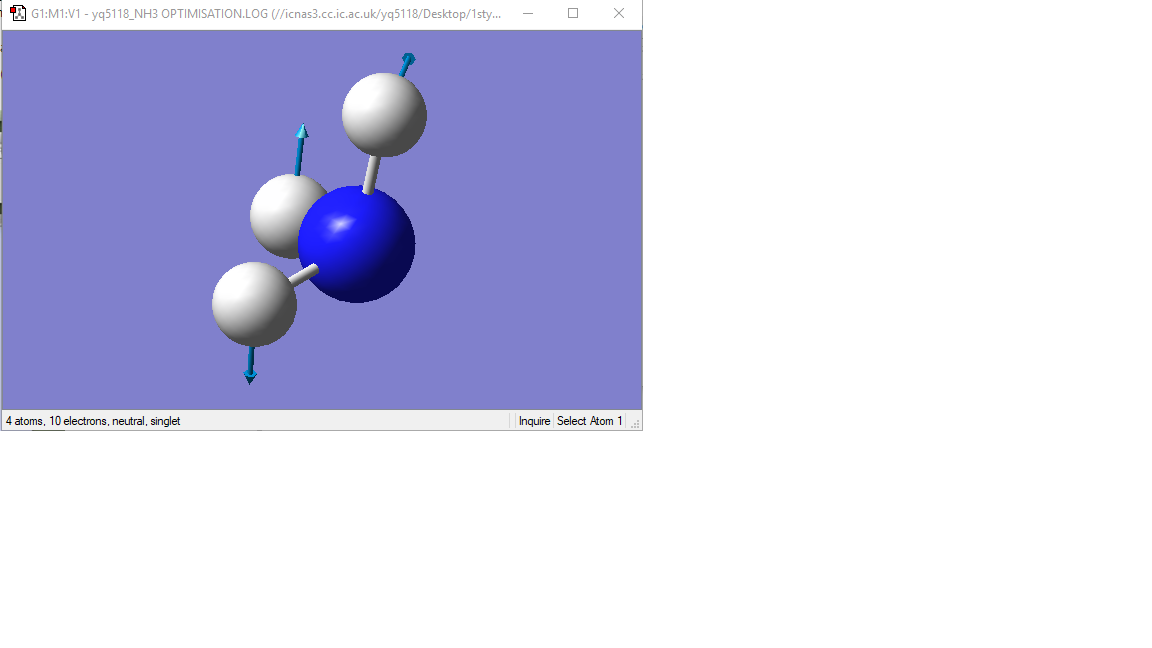
|
| Wavenumber
cm-1 |
1694 | 3461 | |
| Symmetry | E | A1 | |
| Intensity
arbitrary units |
13.55 | 1.061 | |
| Image | 
|
|
| Wavenumber
cm-1 |
3590 | 3590 | |
| Symmetry | E | E | |
| Intensity
Arbitrary units |
0.271 | 0.271 | |
| Image | 
|
|
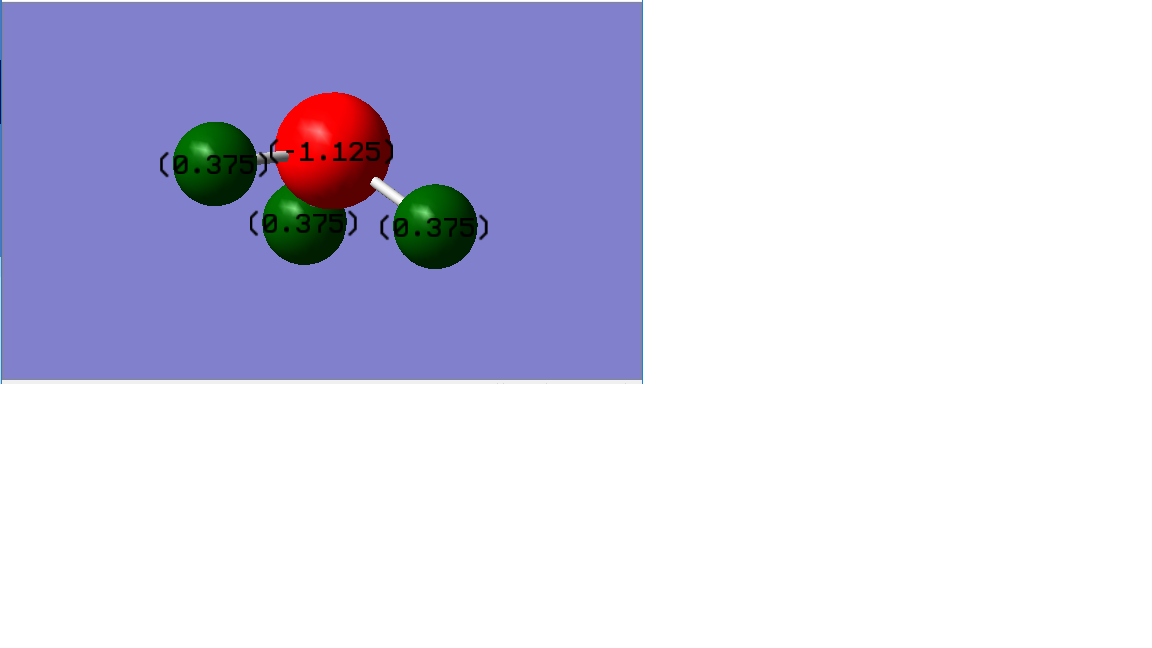
Charge Distribution
Since the nitrogen has higher electronegativity than hydrogen, so the electron pair in the bond is attracted more to the nitrogen. This results in the negative charge in N-atom and positive charge in H-atom. So the red atom is N-atom and has a charge of -1.125. The green atoms are H-atom and all with a charge of 0.375.
H2 Molecule
information of H2
| Name | H2 |
| Calculation type | FREQ |
| Calculation title | RB3LYP |
| Basis set | 6-31G(d,p) |
| E9RB3LYP) | -1.1785394 a.u. |
| RMS gradient | 0.0000002 a.u. |
| Point group | D*H |
optimisation of H2
| Bond length | 0.74279 |
| Bond angle | zero(linear shape) |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.167770D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Jmol dynamic information
test molecule |
You can find the optimised H2 in this link:Media:Yq_H2_OPTIMISATION.LOG
Vibration of H2
basic information about vibration
Vibration mode
| Vibration mode |
|---|
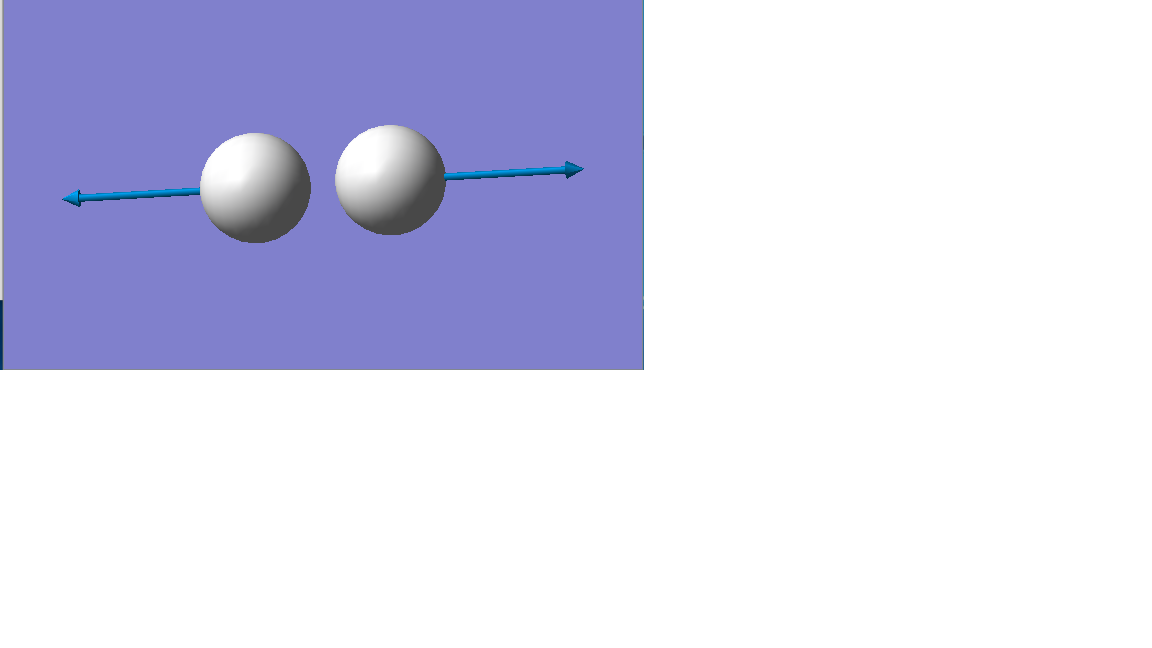
|
| wavenumber/cm-1 |
| 4466 |
| symmetry |
| SGG |
| intensity/arbitrary units |
| 0 |
Charge distribution
Since H2 is a non-polar molecule, so there is no charge on each of the hydrogen atom, so no dipole moment.
N2 Molecule
Information of N2
| Name | N2 |
| Calculation type | FREQ |
| Calculation title | RB3LYP |
| Basis set | 6-31G(d,p) |
| E9RB3LYP) | -109.5241287 a.u. |
| RMS gradient | 0.0000006 a.u. |
| Point group | D*H |
optimisation of N2
| Bond length | 1.1055 |
| Bond angle | zero(linear shape) |
Item table
Item Value Threshold Converged
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401189D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Jmol dynamic range
test molecule |
This can be found in the link:Media:YQ_N2_OPTIMISATION.LOG
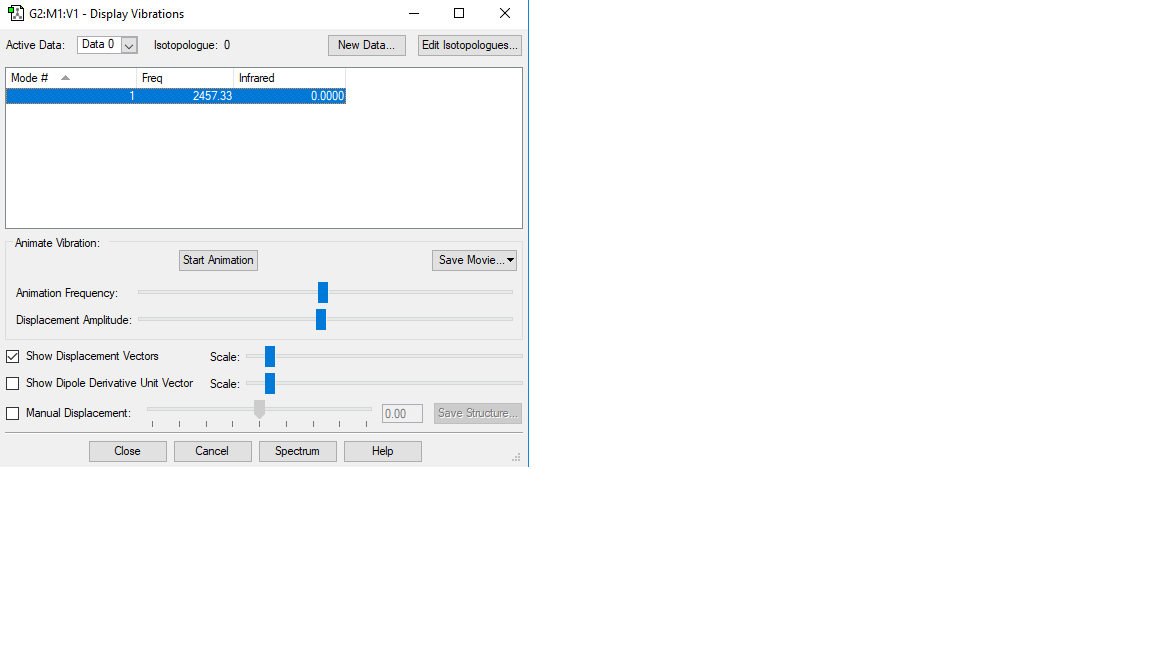
Vibration of N2
Basic information of the vibration of N2
Vibration mode
| Vibration mode |
|---|
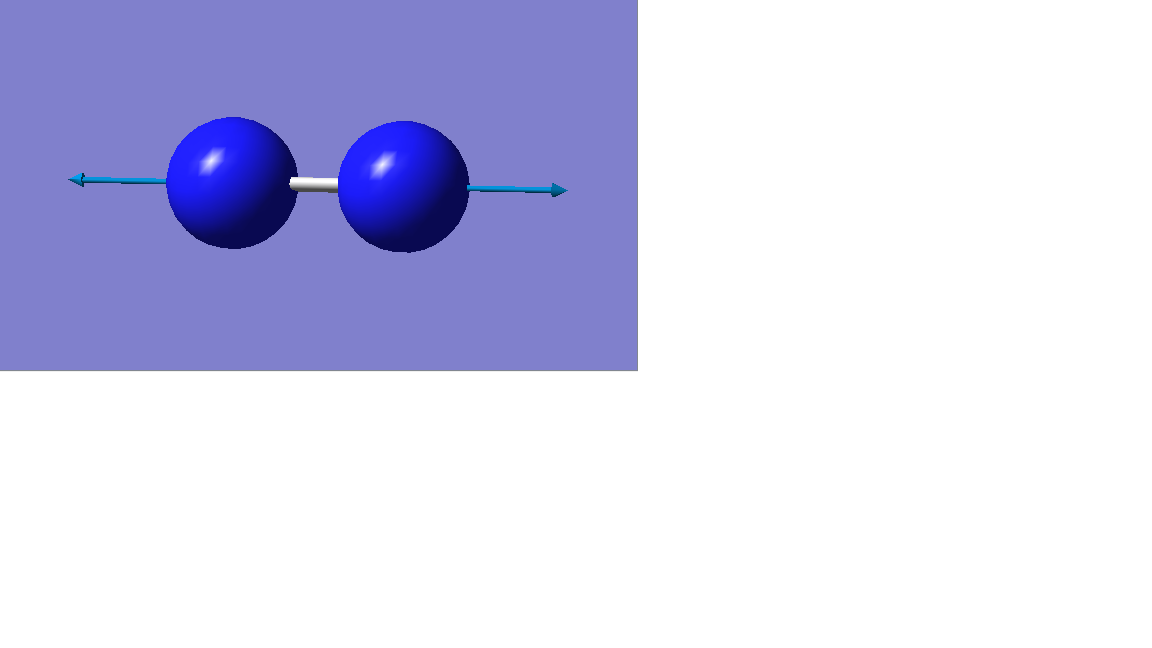
|
| wavenumber/cm-1 |
| 2457 |
| symmetry |
| SGG |
| intensity/arbitrary units |
| 0 |
Charge distribution
Since N2 is a non-polar molecule, so there is no charge on each of the hydrogen atom, so no dipole moment.
The mono-metallic TM complex
The research shown that bis(dinitrogen)-(1,1,4,8,11,11-hexaphenyl-1,4,8,11-tetraphosphaundecane)-molybdenum structure AQUVOY and the two N-N distances are 1.1055 and 1.12(1) Å. The difference is caused by the central metal in the picked complex molecule which forms a bond with the N2 legend. Thus one of the nitrogen is attracted by the metal, increasing the N-N length. Moreover, the bond distance given by computer is the distance in the lowest-energy molecule. But experimentally, molecules are not always in the lowest energy state, this should be accounted for the difference in bond lengths. Additionally, the nitrogen is in gaseous state when calculated by computer, but the complex compounds are not always in gaseous state. These all account for the difference in bond lengths.
Reference to the link https://www.ccdc.cam.ac.uk/structures/search?pid=csd:AQUVOY
Haber-Bosch process
equation
N2 + 3H2 -> 2NH3
Energy information about Haber-Bosch process
E(NH3)=-56.5577687 a.u.
2*E(NH3)=-113.1155375 a.u.
E(N2)=-109.5241287 a.u.
E(H2)=-1.1785394 a.u.
3*E(H2)=-3.5356182 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557906 a.u.=-146.5 KJ/mol
Since product is in higher energy state than the reactants. so the reactants are more stable than ammonia as it's exothermic reaction and heat(energy)is released.
CO Molecule
Information about CO
| Name | CO |
| Calculation type | FREQ |
| Calculation title | RB3LYP |
| Basis set | 6-31G(d,p) |
| E9RB3LYP) | -113.30945314 |
| RMS gradient | 0.00001828 |
| Point group | C*V |
Optimisation of CO
| C=0 bond distance | 1.2584 |
| bond angle | zero(linear shape) |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000032 0.000450 YES
RMS Force 0.000032 0.000300 YES
Maximum Displacement 0.000012 0.001800 YES
RMS Displacement 0.000018 0.001200 YES
Predicted change in Energy=-3.960922D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1379 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Jmol dynamic range
test molecule |
This can be found in the linkMedia:YQ_CO_OPTIMISATIONN.LOG
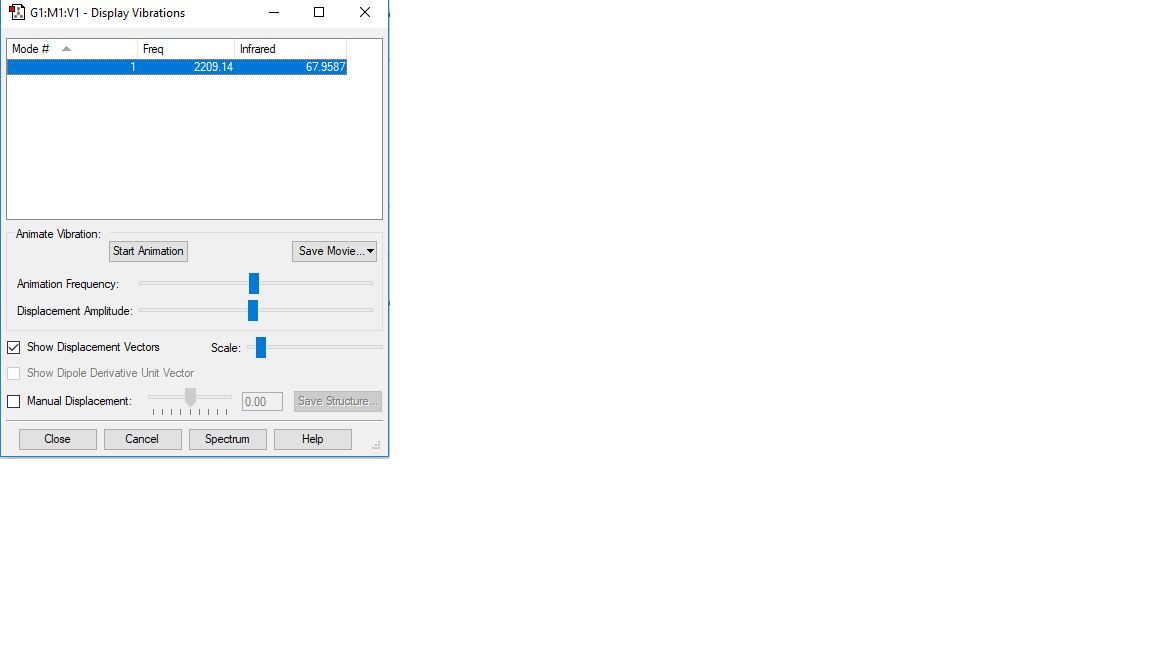
Vibration of CO
basic information of the vibration of CO
Vibration mode
| Vibration mode |
|---|
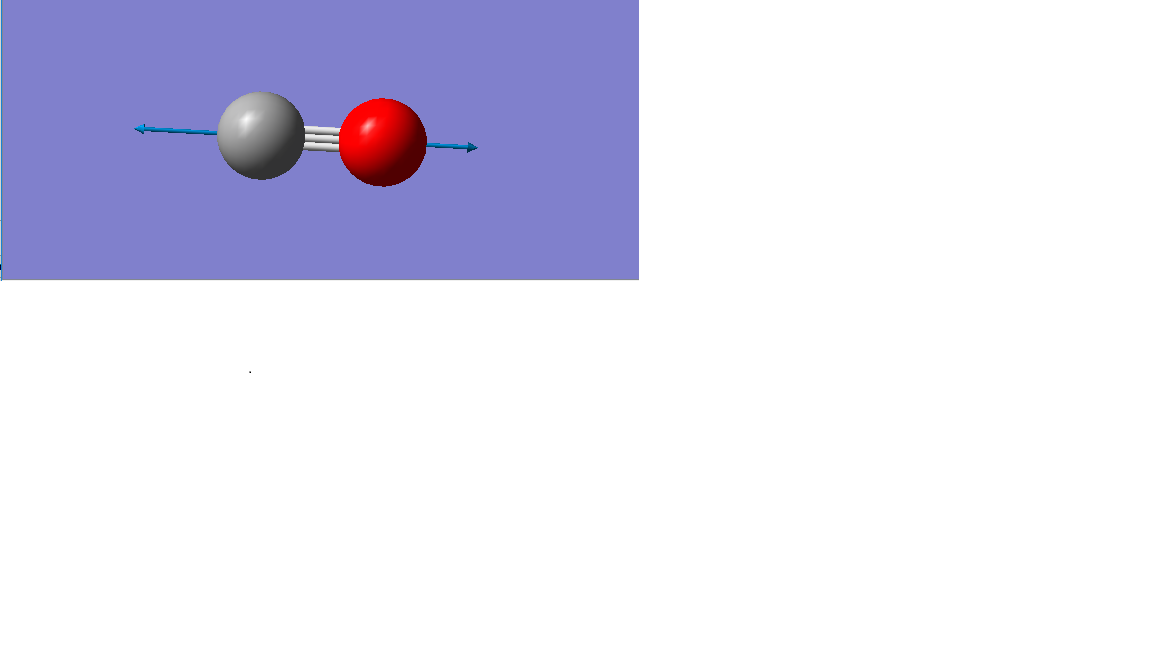
|
| wavenumber/cm-1 |
| 2209 |
| symmetry |
| SGG |
| intensity/arbitrary units |
| 0 |
Charge Distribution
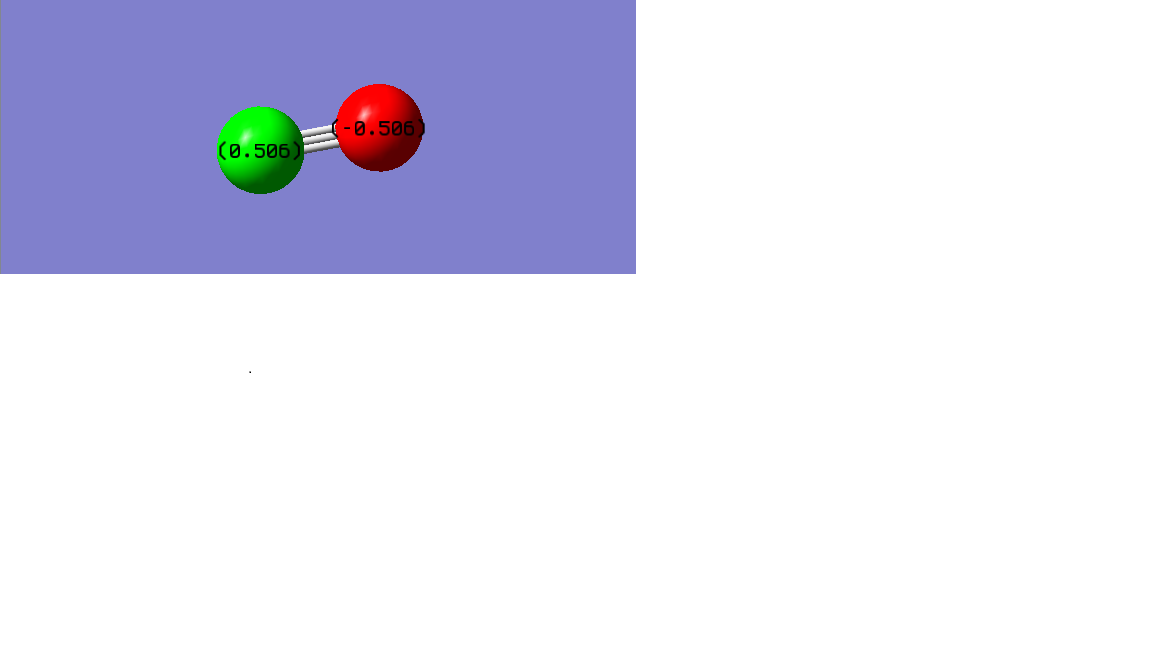
Since the oxygen has higher electronegativity than carbon, so the electron pair in the bond is attracted more to the oxygen. This results in the negative charge in O-atom and positive charge in C-atom. So the red atom is O-atom and has a charge of -0.506. The green atoms are C-atom and all with a charge of 0.506.
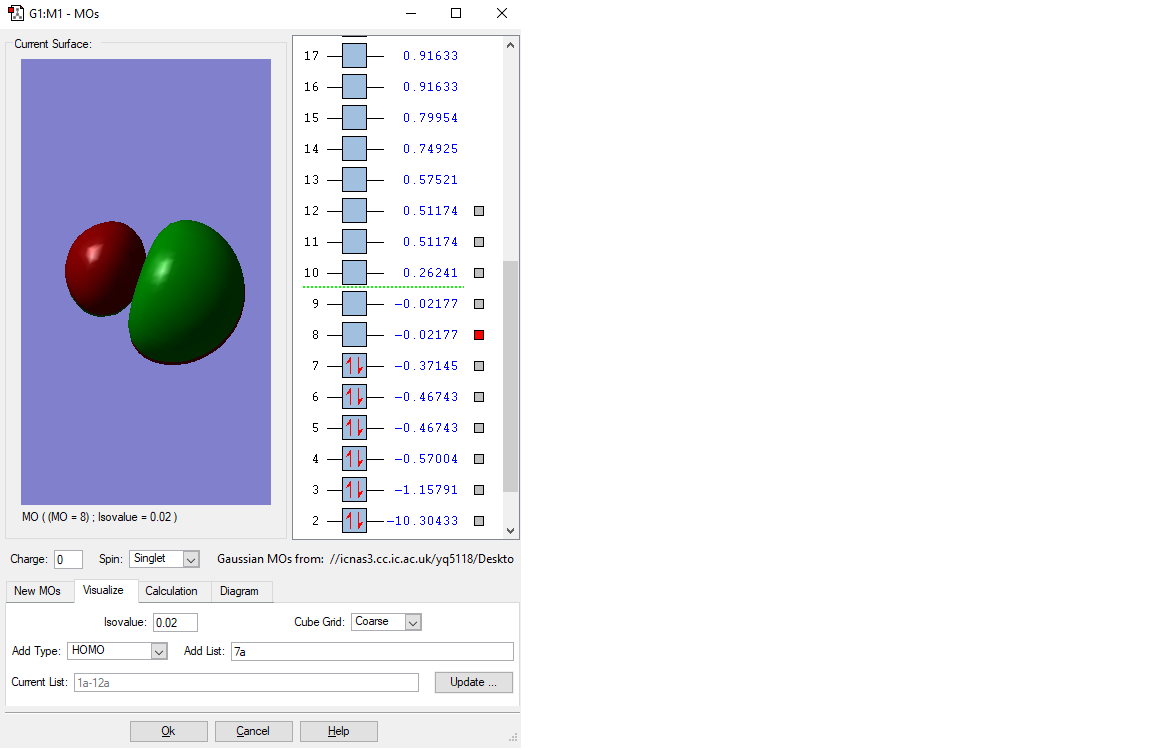
Molecular orbital diagrams of CO
The electronic configuration of a O-atom is: 1s22s22p4, the electronic configuration of a C is 1s22s2. Different molecular orbitals of CO is formed from the overlap of different atomic orbitals in the C and O orbitals.
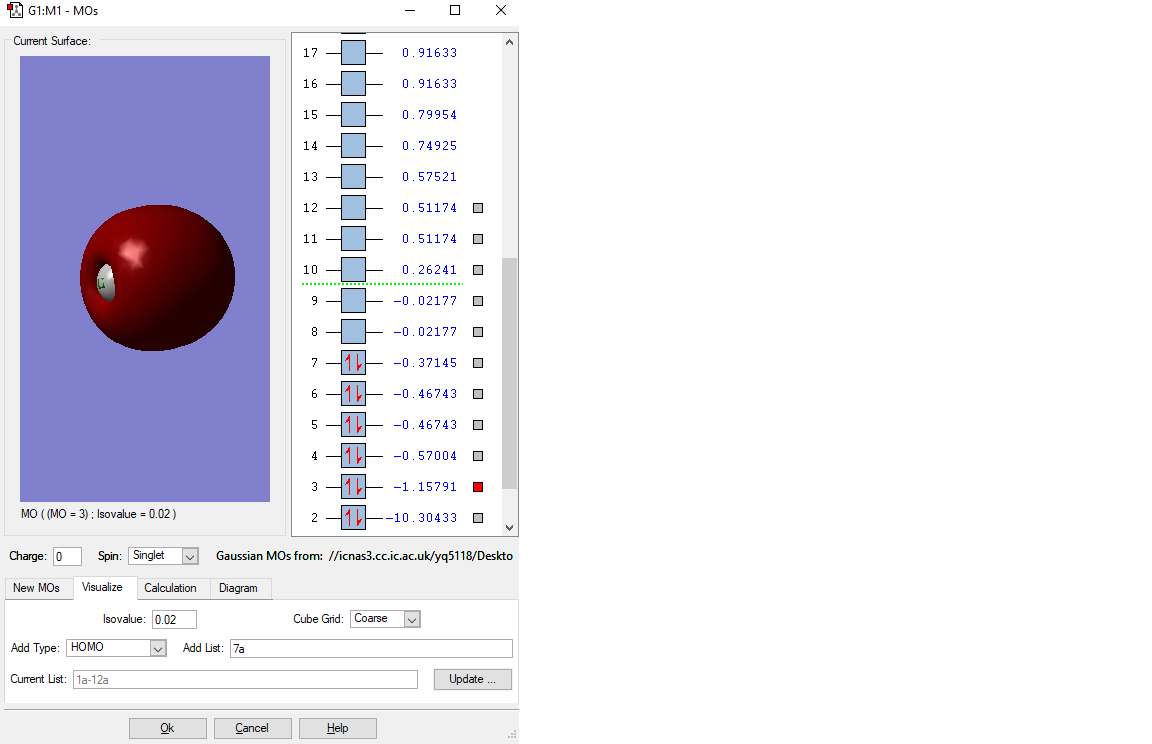
MO of 1s bonding orbital
 This is the two atomic orbitals of O atom and C atom in the lowest energy state, which has an orbital energy of -19.25805(a.u.). Moreover, the orbitals of atoms do not have overlap, no formation of MO.
Because the outer lower energy electrons will first be used in the reaction. Thus, this orbital is so deep in energy(close to the nucleus) and thus will not be involve in the chemical reaction.
This is the two atomic orbitals of O atom and C atom in the lowest energy state, which has an orbital energy of -19.25805(a.u.). Moreover, the orbitals of atoms do not have overlap, no formation of MO.
Because the outer lower energy electrons will first be used in the reaction. Thus, this orbital is so deep in energy(close to the nucleus) and thus will not be involve in the chemical reaction.
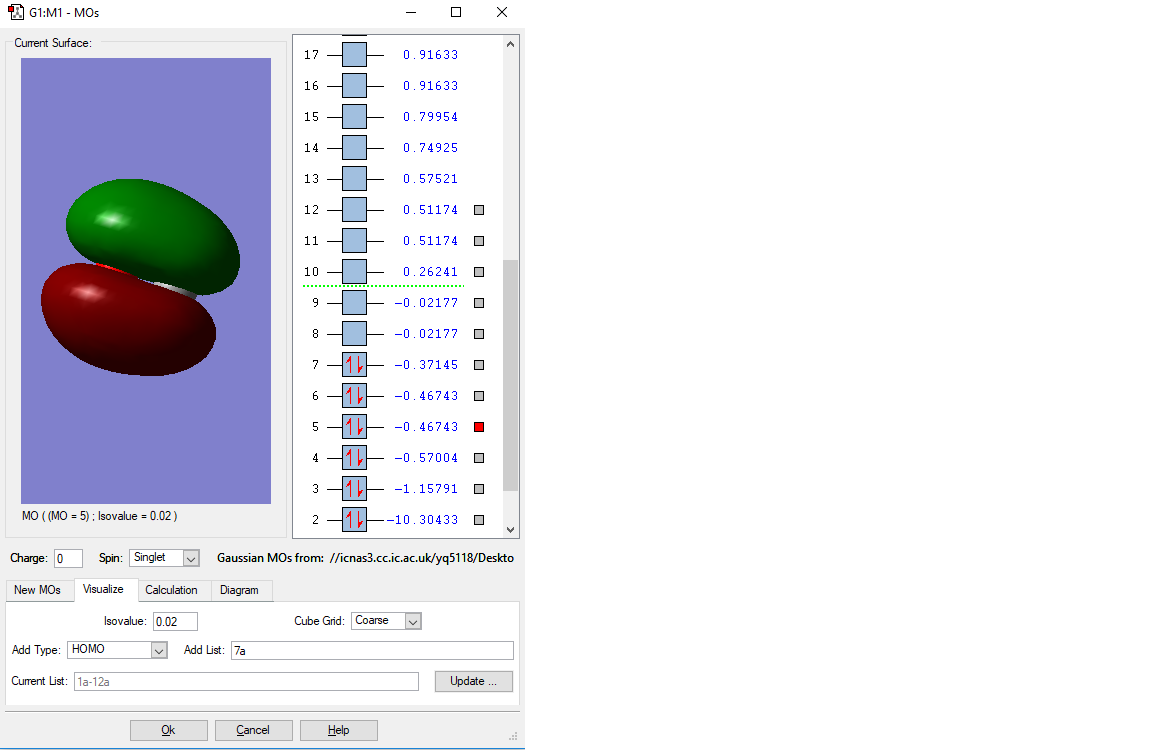
MO of 2s bonding orbital
This is the 2s bonding MO of the CO molecule. It is fromed by the overlap of two 2s orbitals from the O and C atom. It has a higher energy(-1.15791 a.u.) than the upper 1s one, but it is still very low in energy compared with many other orbitals and is thus be occupied. Because CO molecule is SP hybridisation. This orbital is thought to be the s orbital of this hybridisation, as it is in the highest energy level among s orbitals. It will be easier to be involve in the chemical reactions.
MO of 2p bonding orbital
This is the bnding orbital of 2p orbital. And this is formed by the overlap of two atomic p orbitals from the O and C atom. It has a orbital energy of -0.46743 a.u.. It is noticed that there is another MO which has the same energy with this orbital. This is because each O atom has two unhybridised p orbitals, the overlap between the two unhybridised p orbitals results in the two degenerate energy levels. This orbital is in higher energy level and even close to the HOMO-LUMO region. Thus it is very possibly be involved in the chemical reaction.
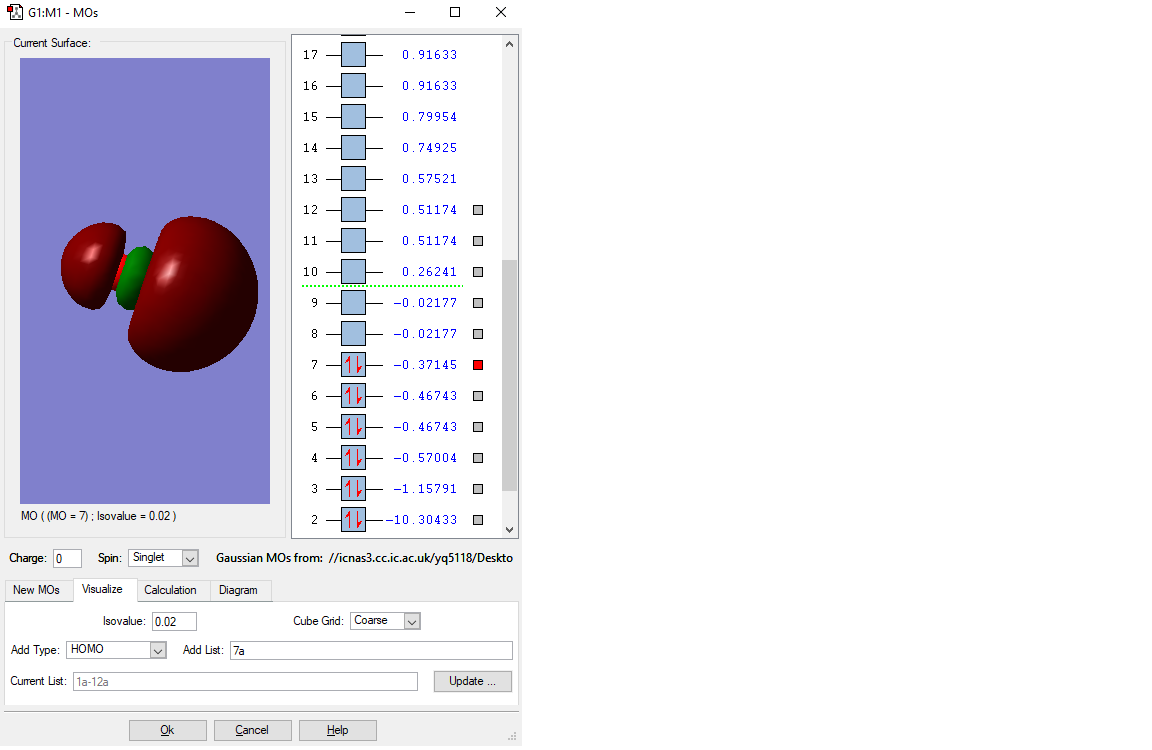
MO of the HOMO
This is the HOMO(highest occupied molecular orbital) of CO molecule. This is the anti-bonding of the two unhybridised p orbitals in O and C atom, and this has a negative contribution to bonding, thus forming two anti-bonding with the same energy levels. So the two orbitals are both thought to be the HOMO. They both have -0.37145a.u. orbital energy. The difference between the two MOs is direction.
Because they are HOMOs and has high energy, it is usually involved in the chemical reactions. Especially in the reactions having exchanges of electrons.
MO of the LUMO
This is the LUMO(lowest unoccupied molecular orbital). This is also an anti-bonding orbital and with no electron occupied in this orbital. Because it is so high in energy(-0.02177 a.u.), it can be involved most easily in the chemical reactions. This orbital is formed when two hybridised p orbitals overlap. Because of hybridisation, the anti-bonding will be hybridised to have higher energy than other anti-orbitals formed by two unhybridised p orbitals.
O2 molecule
basic information
| Name | BF3 |
| Calculation type | FREQ |
| Calculation title | RB3LYP |
| Basis set | 6-31G(d,p) |
| E9RB3LYP) | -150.25250603 a.u. |
| RMS gradient | 0.05905207 a.u. |
| Point group | D*H |
optimisation of O2
| O=O bond distance | 1.1616 |
| N-H-N bond angle | ZERO(linear shape) |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000130 0.000450 YES
RMS Force 0.000130 0.000300 YES
Maximum Displacement 0.000080 0.001800 YES
RMS Displacement 0.000112 0.001200 YES
Predicted change in Energy=-9.882887D-09
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.216 -DE/DX = -0.0001 !
--------------------------------------------------------------------------------
Jmol dynamic Range
test molecule |
This can be found in this link:Media:YQ_O2_OPTIMISATIONN.LOG
Vibration of O2
basic information of vibration
Vibration mode
| Vibration mode |
|---|
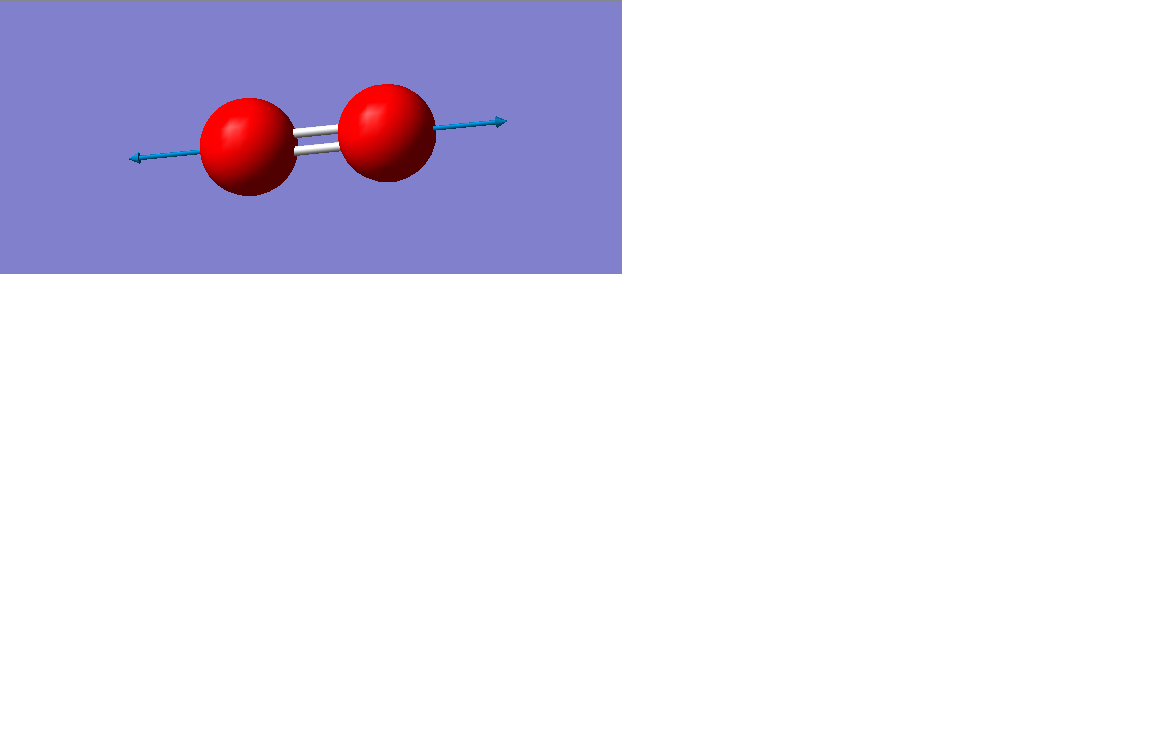
|
| wavenumber/cm-1 |
| 1643 |
| symmetry |
| SGG |
| intensity/arbitrary units |
| 0 |
Charge Distribution
Since O2 is a non-polar molecule, so there is no charge on each of the oxygen atom, so no dipole moment.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES - however, all your jmols are labelled 'test molecule' which is not informative at all. Your tables could have been arranged more clearly.
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - you correctly described degenerate modes and their influence on the number of expected bands. You missed to analyse the relative intensities of the different modes. Modes 4, 5 and 6 are too low in energy too be seen in an experimental spectrum. Therefore, only 2 bands are expected.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES - however, you stated a bond angle for diatomic molecules. To define a bond angle a minimum of 3 atoms is needed!
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES - however, the energy of ammonia is lower in energy than the sum of the energies of the reactants (which you correctly calculated and can be seen on the negative sign of the reaction energy). Therefore, ammonia is more stable than the reactants.
Your choice of small molecule 3.5/5
Have you completed the calculation and included all relevant information?
YES - A typo occurred, the heading of the table regarding computational details claims you calculated Cl2 but the rest of this section is based on CO. You could have explained the calculated vibration of CO briefly.
Have you added information about MOs and charges on atoms?
YES
The fist displayed MO is no combination of the 1s orbitals of C and O. This is only the 1s of O. The 1s orbital of C can be found in the MO with the energy of 10.30433 a.u. As you correctly stated it is not involved in bonding and therefore should be called non-bonding. Just as additional information to the description of the 2nd displayed MO: A minor contribution of 2p orbitals can be identified by the small area with a different phase (the small green part) than the rest of the MO. The HOMO is a bonding MO rather than an anti-bonding one because there is no node along the bond. You stated the HOMO to be generated but as one can see from the screenshot the orbitals next to the HOMO are degenerate and not the HOMO itself. You missed to report the occupancy of the MOs except for the HOMO and LUMO. For future reports try to be as specific as you can. You correctly stated the contributing AOs but could have made more clearly how the p-orbitals are oriented (px, py and pz) and how bonding/anti-bonding orbitals are formed (by in-phase or out-of-phase overlap). You could have commented on the relative MO energies by looking at the number of nodes etc.
Independence 0.5/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES - You are mixing up some different molecules in this section. You calculated O2 but you are reporting BF3 several times in your report. Additionally, you stated a NHN bond angle even though your molecule does not contain N nor H. Probably most of these mistakes result by copying and pasting from previous sections but they are making it harder to follow this section.
Do some deeper analysis on your results so far