Rep:Mod:ym2814
Introduction
Transition state is very important for the studying of a reaction. Gaussview can be used to help locate the transition states for different reaction route (eg. Endo- or Exo). From the results, the energy of each states can be clearly seen and therefore help to calculate the activation energy and analyse the thermodynamically and kinetically favoured product.
Nf710 (talk) 23:33, 17 November 2016 (UTC) You have explained what a TS is or how to find it.
Exercise 1: Reaction of Butadiene with Ethylene
- MO diagram
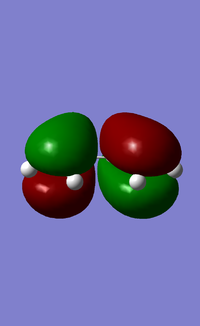
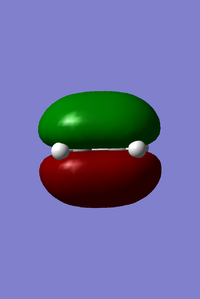
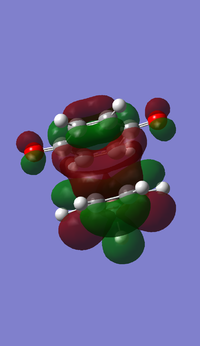
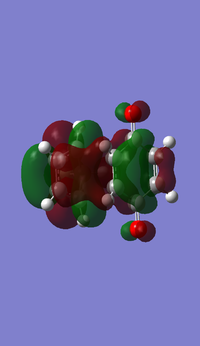
- HOMO and LUMO of butadiene
- HOMO and LUMO of ethene
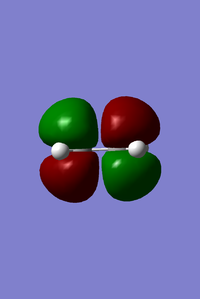
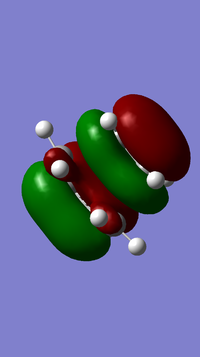
- MOs of transition state
From left to right, the energy of MO goes up as shown in the MO diagram. The HOMO of butadiene interacts with LUMO of ethene to form two gerade MOs and LUMO of butadiene interacts with HOMO of ethene to form two ungerade MOs.The former one is favoured due to smaller energy gap. Two MOs with same symmetry can interact with each other to form two MOs with unchanged symmetry. The reaction is allowed when interactions between MOs with same symmetry occur and is forbidden for ungerade-gerade interactions. The overlap integral is non-zero for gerade-gerade interaction and ungerage-ungerade interaction while the overlap integral is zero for gerade-ungerade interaction.
- Bond lengths
- 1. C-C bond lengths of transition state
| C1-C2 | partly formed C2-C3 | C3-C4 | C4-C5 | C5-C6 | partly formed C6-C1 | |
|---|---|---|---|---|---|---|
| Bond length/Å | 1.382 | 2.115 | 1.380 | 1.411 | 1.380 | 2.115 |
- 2. C-C bond lengths of product
| C1-C2 | C2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-C1 | |
|---|---|---|---|---|---|---|
| Bond length/Å | 1.540 | 1.540 | 1.500 | 1.338 | 1.500 | 1.540 |
From transition state to product, the bond length for ethene (C1-C2) increases since it change from double bond to single bond and as for the bond lengths for butadiene, two original double bonds change into single bonds so the bond lengths increases and the original single bond becomes double bond so the bond length decreases. Two new single bonds (C2-C3, C1-C6) are formed.
- 3. typical bond length[1]
| sp3-sp3 | sp3-sp2 | C=C double bond | |
|---|---|---|---|
| Bond length/Å | 1.540 | 1.500 | 1.340 |
The Van der Waals radius of the C atom is 1.70Å[2]. The lengths of the partly formed C-C bonds in the TS are both 2.115Å, which means bonds are forming between those two pairs of carbons (C2-C3,C6-C1) because the length of partly formed bond is smaller than the shortest distance between two non-bonded carbon atoms.
- Vibrations
According to the results, the formation of two bonds are synchronous and the frequency is negative. As for the lowest positive frequency, the formation of two bonds are asynchronous.
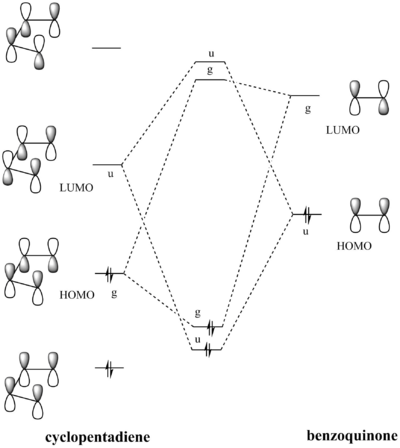
Exercise 2: Reaction of Benzoquinone with Cyclopentadiene
- MO diagram
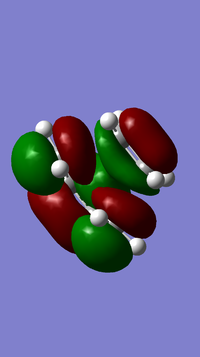
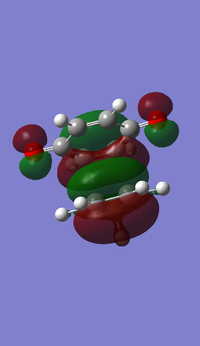
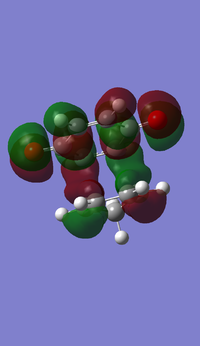
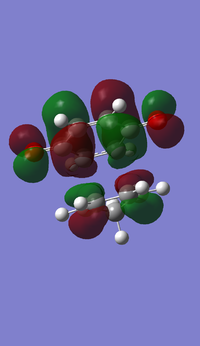
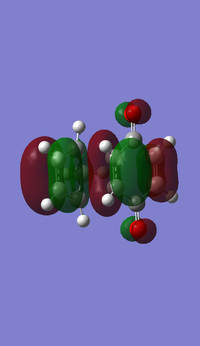
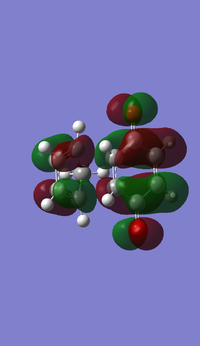
- MOs for Endo TS
- MOs for Exo TS
(This angle makes it quite difficult to see what's going on, but it does show the symmetry. In addition, you should label the diagram to make it explicitly clear which orbitals are which Tam10 (talk) 13:01, 9 November 2016 (UTC))
The MOs show that the interaction between LUMO of cyclopentadiene and HOMO of benzoquinonethe is favoured, therefore the reaction is inverse demand DA reaction.
- Activation Energy and Reaction Energy
- 1. Endo
| reactants | transition state | product | |
|---|---|---|---|
| sum of electronic and thermo free energies/ KJmol-1 | 0.130712 | 0.180247 | 0.108079 |
activation energy= 0.180247-0.130712= 0.049535 KJmol-1
reaction energy= 0.108079-0.130712= -0.022633 KJmol-1
- 2. Exo
| reactants | transition state | product | |
|---|---|---|---|
| sum of electronic and thermo free energies/ KJmol-1 | 0.130712 | 0.181212 | 0.107565 |
activation energy= 0.181212-0.130712= 0.050500 KJmol-1
reaction energy= 0.017565-0.130712= -0.023147 KJmol-1
The endo one is the kinetically favoured product due to lower activation energy and the exo one is the thermodynamically favoured because the product has lower energy and therefore more stable.
In the most cases, the exo product is thermodynamically more stable due to less steric hindrance while the endo product forms more rapidly (kinetic control) because of secondary orbital interactions[3].
According to the HOMOs of TSs, there is secondary orbital interactions between the double bonds of cyclopentadiene and the other side of the benzoquinone (not the side of reaction) in the endo one but not in the exo one, which results in stable endo transition state and therefore lower reaction barrier energy.
Nf710 (talk) 23:43, 17 November 2016 (UTC) This section was done ok. you could have talked about the significance of the imaginary frequency and what it means. Also you could have used Jmols and labeled better
Nf710 (talk) 23:56, 17 November 2016 (UTC)I dont know what energies you have used here, but they are way off and certainly not kJmol-1. Hence you have got the wrogn conclusion. Your reasoning for the kenetic product is good however. Next time use Jmols the angle was hard to see what was going on.
Exercise 3: Diels-Alder vs Cheletropic
- Reaction Coordinate
- 1. Diels-Alder
(There are two DA reactions, an endo and an exo Tam10 (talk) 13:01, 9 November 2016 (UTC))
- 2. Cheletropic
- Activation Energy and Reaction Energy
- 1. Diels-Alder
| reactants | transition state | product | |
|---|---|---|---|
| sum of electronic and thermo free energies/ KJmol-1 | 0.067654 | 0.092078 | 0.021455 |
(The energies provided by Gaussian are in Hartrees, not kJ/mol! 1 Hartree = 2625 kJ/mol. You should notice that the numbers you've given are very low for reactions Tam10 (talk) 13:01, 9 November 2016 (UTC))
activation energy= 0.092078-0.067654= 0.024424 KJmol-1
reaction energy= 0.021455-0.067654= -0.046199 KJmol-1
- 2 Cheletropic
| reactants | transition state | product | |
|---|---|---|---|
| sum of electronic and thermo free energies/ KJmol-1 | 0.067939 | 0.099060 | 0.000002 |
activation energy= 0.099060-0.067939= 0.031121 KJmol-1
reaction energy= 0.000002-0.067939= -0.067937 KJmol-1
The Diels-Alder reaction has lower activation energy therefore it is the kinetically preferred route and the cheletropic product has much lower energy so it is the thermodynamically favoured route.
(You need to have a reaction profile comparing the reactions Tam10 (talk) 13:01, 9 November 2016 (UTC))
During both reactions, reasonaces within the 6-membered ring can be seen, which lower the energy ad make the o-xylylene more stable.
Conclusion
For each set of reactions, the thermodynamicaly preferred product is the one with lower energy product and the kinetically favoured product is the one with lower activation energy. As for Diels-Alder reaction, the endo product is always the kinetically preferred product due to secondary orbital interactions and the exo one is the thermodynamically favoured.
References
- ↑ Andreas A. Zavitsas, "The Relation between Bond Lengths and Dissociation Energies of Carbon-Carbon Bonds", J. Phy. Chem. A, 2003, 107, 897-898.DOI:10.1021/jp0269367
- ↑ Alvarez Santiago, "A Cartography of the Van der Waals Territories", Dalton Trans, 2013, 42, 8617-8636.DOI:10.1039/C3DT50599E
- ↑ Cooley, James H., Williams, Richard Vaughan, "Endo- and Exo-Stereochemistry in the Diels-Alder Reaction: Kinetic versus thermodynamic control", Journal of Chemical Education, May 1997, Vol.74(5), pp.582-585.DOI:10.1021/ed074p582