Rep:Mod:yixingphysical
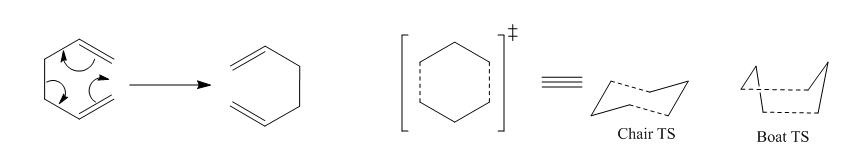
Cope Rearrangement
Introduction
1,5-hexadiene could undergo a [3,3]-sigmatropic cope rearrangement as shown below. The mechanism would be via transition state either 'chair' or 'boat'. Enthalpies and energies of both transition states were computed and compared in order to decide the mechanism of the cope rearrangement.
Optimizations of Reactants and Products
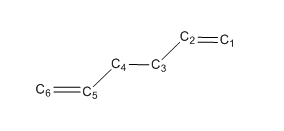
Optimizations of Anti-Peri Planar Conformer of 1,5-hexadiene
1. Optimized Anti Structure
Pentahelicene |
2. Key Information of Result
| Molecule | Anti 1,5-hexadiene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | HF/3-21G |
| Total Energy | -231.69253528 a.u. |
| Charge | 0 |
| Gradient | 0.00001891 |
| Dipole Moment | 0.0000 |
| Point Group | Ci |
| Jop cpu Time | 0 days 0 hours 0 minutes 32.0 seconds |
3. Validity of Result
Item Value Threshold Converged?
Maximum Force 0.000060 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000457 0.001800 YES
RMS Displacement 0.000171 0.001200 YES
Predicted change in Energy=-2.036873D-08
Optimization completed.
-- Stationary point found.
To check if the job has been successfully converged, an 'Item' table is shown above. It tells what forces have been converged. Also, according to the table, all gradient values were quite close to zero, ie less than 0.001; hence, the optimization job was complete.
4.File Link
To access the .log file of optimization, click anti 1,5-hexadiene 3-21G.
Re-optimizations of Anti-Peri Planar Conformer of 1,5-hexadiene
1.Optimized Anti Structure
Pentahelicene |
2. Key Information of Results
| Molecule | Anti 1,5-hexadiene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Total Energy | -231.61170280 a.u. |
| Charge | 0 |
| Gradient | 0.00001326 |
| Dipole Moment | 0.0000 |
| Point Group | Ci |
| Jop cpu Time | 0 days 0 hours 0 minutes 9.0 seconds |
3. Validity of Result
Item Value Threshold Converged?
Maximum Force 0.000015 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000219 0.001800 YES
RMS Displacement 0.000079 0.001200 YES
Predicted change in Energy=-1.588856D-08
Optimization completed.
-- Stationary point found.
4. Frequency Analysis
Sum of electronic and zero-point Energies at 0 K (Hartrees) = -234.469212 Sum of electronic and thermal Energies at 298.15 K (Hartrees) = -234.461856
Item Value Threshold Converged?
Maximum Force 0.000036 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000228 0.001800 YES
RMS Displacement 0.000105 0.001200 YES
Predicted change in Energy=-1.497856D-08
Optimization completed.
-- Stationary point found.
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
5.File Link
To access the .log file of optimization, click here; for the .log file of frequency analysis, click here.
Optimizations of Gauche (3) Conformer of 1,5-hexadiene
1. Optimized Gauche Structure
Pentahelicene |
2. Key Information of Results
| Molecule | Gauche 1,5-hexadiene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Total Energy | -231.69266122 a.u. |
| Charge | 0 |
| Gradient | 0.00000702 |
| Dipole Moment | 0.3405 |
| Point Group | C1 |
| Jop cpu Time | 0 days 0 hours 0 minutes 29.0 seconds |
3. Validity of Results
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000934 0.001800 YES
RMS Displacement 0.000298 0.001200 YES
Predicted change in Energy=-8.790932D-09
Optimization completed.
-- Stationary point found.
4.File link
To access the .log file of optimization, click here
Re-optimizations of Gauche(3) Conformer of 1,5-hexadiene
1. Optimized Gauche Structure
Pentahelicene |
2. Key Information of Results
| Molecule | Gauche 1,5-hexadiene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Total Energy | -231.61132934 a.u. |
| Charge | 0 |
| Gradient | 0.00000382 |
| Dipole Moment | 0.1383 |
| Point Group | C1 |
| Jop cpu Time | 0 days 0 hours 1 minutes 30.0 seconds |
3. Validity of Results
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000537 0.001800 YES
RMS Displacement 0.000134 0.001200 YES
Predicted change in Energy=-1.500532D-09
Optimization completed.
-- Stationary point found.
4. Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000668 0.001800 YES
RMS Displacement 0.000177 0.001200 YES
Predicted change in Energy=-1.980461D-09
Optimization completed.
-- Stationary point found.
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Sum of electronic and zero-point Energies at 0 K (Hartrees) = -234.468693 Sum of electronic and thermal Energies at 298.15 K (Hartrees) = -234.461464
5.File Link
To access the .log file of optimization, click here; for the .log file of frequency analysis, click here.
Optimizations of Gauche (4) Conformer of 1,5-hexadiene
1. Optimized Gauche Structure
Pentahelicene |
2. Key Information of Results
| Molecule | Gauche 1,5-hexadiene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Total Energy | -231.69153034 a.u. |
| Charge | 0 |
| Gradient | 0.00001325 |
| Dipole Moment | 0.1280 |
| Point Group | C2 |
| Jop cpu Time | 0 days 0 hours 0 minutes 4.0 seconds |
3. Validity of Results
Item Value Threshold Converged?
Maximum Force 0.000021 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.001439 0.001800 YES
RMS Displacement 0.000540 0.001200 YES
Predicted change in Energy=-2.875776D-08
Optimization completed.
-- Stationary point found.
4.File link
To access the .log file of optimization, click here
Re-optimizations of Gauche Conformer of 1,5-hexadiene
1. Optimized Gauche Structure
Pentahelicene |
2. Key Information of Results
| Molecule | Gauche 1,5-hexadiene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Total Energy | -231.61048196 a.u. |
| Charge | 0 |
| Gradient | 0.00001090 |
| Dipole Moment | 0.1383 |
| Point Group | C2 |
| Jop cpu Time | 0 days 0 hours 0 minutes 47.0 seconds |
3. Validity of Results
Item Value Threshold Converged?
Maximum Force 0.000021 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000457 0.001800 YES
RMS Displacement 0.000144 0.001200 YES
Predicted change in Energy=-1.213327D-08
Optimization completed.
-- Stationary point found.
4. Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000022 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000450 0.001800 YES
RMS Displacement 0.000180 0.001200 YES
Predicted change in Energy=-1.181951D-08
Optimization completed.
-- Stationary point found.
GradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGradGrad
Sum of electronic and zero-point Energies at 0 K (Hartrees) = -234.467784 Sum of electronic and thermal Energies at 298.15 K (Hartrees) = -234.460521
5.File Link
To access the .log file of optimization, click here; for the .log file of frequency analysis, click here.
Results and Discussion
1.Symmetry Comparison
| Conformer | Point Group | Literature Point Group |
| Anti 2 | Ci | Ci |
| Gauche 3 | C1 | C1 |
| Gauche 4 | C2 | C2 |
According to the table above, all optimization jobs have been successfully finished because they all generated the correct symmetry point groups. It should be noticed that all point groups were C1 if the log file was opened; this was due to the feature of the program which altered the symmetry in order to have the minimum energy.
2.Thermochemistry Comparison
| Type of energy | Anti-2 B3LYP/631G* | Gauche-3 B3LYP/631G* | Gauche-4 B3LYP/631G* |
| The Sum of Electronic and Zero-Point Energies at 0 K (Hartrees) | -234.469212 | -234.468693 | -234.467784 |
| The Sum of Electronic and Thermal Energies at 298.15 K (Hartrees) | -234.461856 | -234.461464 | -234.460521 |
| The Sum of electronic and thermal Enthalpies | -234.460912 | -234.460520 | -234.459577 |
| The Sum of electronic and thermal Free Energies | -234.500821 | -234.500105 | -234.498690 |
| Total Electronic Energy | -231.61170280 | -231.61132934 | -231.61048196 |
Energies and enthalpies of different conformers using B3LYP/631G* were listed in the above table. Although each conformer was investigated using both B3LYP/631G* and HF/321G basis sets, only the results from B3LYP/631G*(the higher level) were discussed here because energies between diffferent method and basis set cannot be compared.
By comparing the energies and enthalpies, it could be concluded that anti-conformer is more stable than the gauche conformers since anti-conformer has relatively lower energy.
3.Geometry Comparison
The geometry information of the anti-conformer is listed in the following table. Both bond length and bond angles including dihedral angles using two different method and basis set are compared with literature values.
| Terms | HF/3-21G | B3LYP/6-31G* | Literature[1] |
| C1=C2 Bond Length/ Å | 1.3162 | 1.3335 | 1.3412 |
| C2-C3 Bond Length/ Å | 1.5088 | 1.5042 | 1.5077 |
| C3-C4 Bond Length/ Å | 1.5530 | 1.5481 | 1.5362 |
| C-H Bond Length/ Å | 1.075 | 1.0997 | 1.1077 |
| C2-C3-C4 Bond Angle | 111.3465o | 112.6749o | 111.0o |
| C1=C2-C3 Bond Angle | 124.8129o | 121.8691o | 122.5o |
| C2=C1-H Bond Angle | 121.8245o | 121.6515o | 120.4o |
| C3-C2-H Bond Angle | 119.6735o | 118.981o | 118.4o |
| C2-C3-C4-C5 Dihedral Angle | -179.9889o | -180.0000o | -178.3o |
It could be seen that B3LYP/6-31G* method was much more accurate than the HF/3-21G method because the bond length and bond angles were more close to the literature value, and also the dihedral angles of C2, C3, C4 and C5 was exactly 180o.
Optimization of an Allyl Fragment
1. Optimized Allyl Fragment
Pentahelicene |
| Method | HF/3-21G |
| Total Energy | -115.82304010 a.u. |
Optimization of the 'chair' Transition State
1,5-hexadiene undergoes the cope rearrangement via the chair or boat transition state. For chair transition state, the mechanism could be considered as the migration of one allyl fragment.
In order to do this, the CH2CHCH2 fragment was optimized using HF/3-21G method and was used as the basis. Then the transition state was made up with two optimized allyl fragments with a distance of 2.2Å between two terminal carbons. .The chair TS has been investigated using three different approaches: normal optimization, frozen coordinate method and calculation with derivative.
First Approach: Optimization to a TS (Berny)
In the first approach of optimization of the transition state, the Berny algorithm was used. This method is the most time-saving but sometimes might lead to inaccurate results.
1. Optimized Chair Transition State
Pentahelicene |
2. Key Information of Result
| Type of Transition State | Chair |
| Method | Optimization to a TS (Berny) |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | HF/3-21G |
| Keyword | Opt=NoEigen |
| Total Energy | -231.61932247 a.u. |
| Number of Imaginary Frequency | 1 |
| Wavenumber of Imaginary Frequency | -817.93 cm-1 |
| Point Group | C2h |
3.Vibration Mode of Imaginary Frequency
4. File Link
To access to the .log file of optimization, click here
5. Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000486 0.001800 YES
RMS Displacement 0.000122 0.001200 YES
Predicted change in Energy=-8.556510D-09
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energies at 0 K (Hartrees)= -231.466700 Sum of electronic and thermal Energies at 298.15 K (Hartrees)= -231.461340
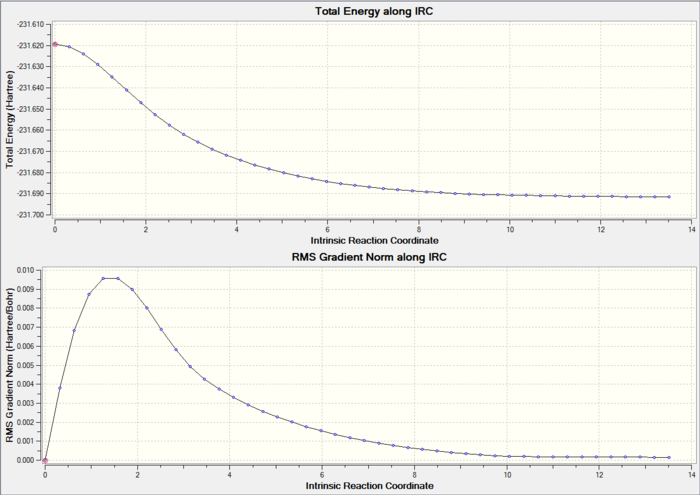
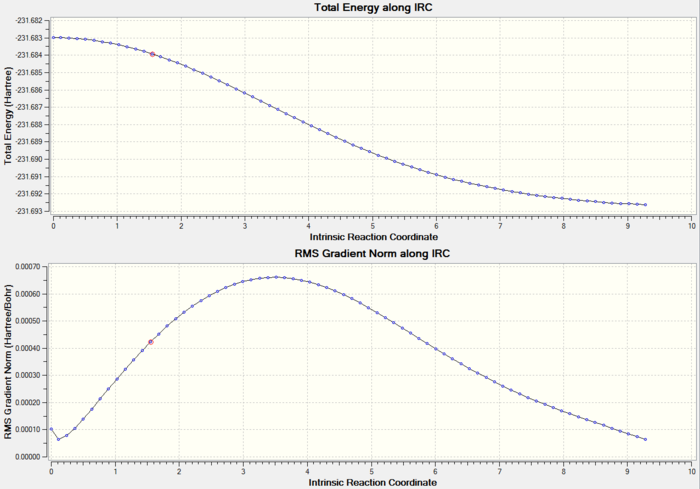
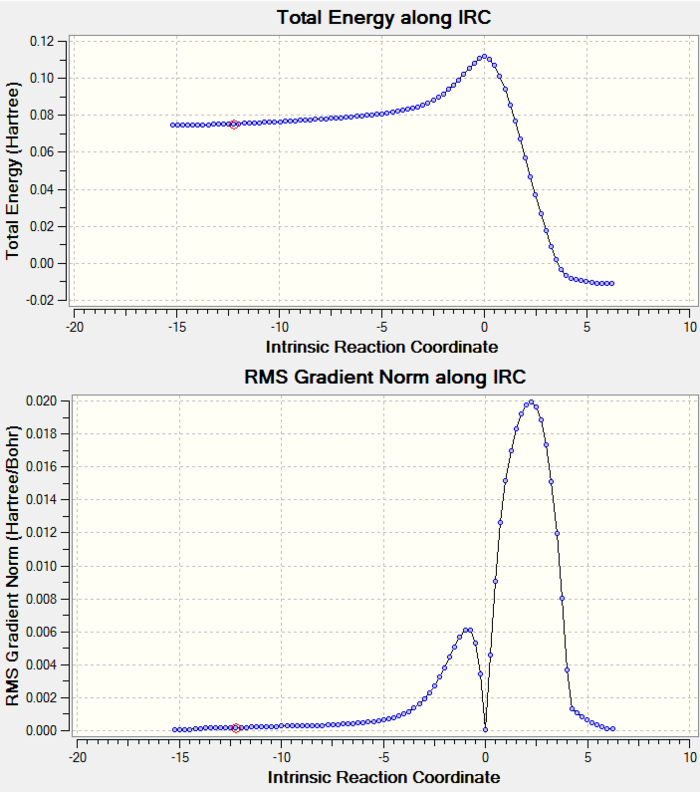
6. IRC Calculation
| Type of Transition State | Chair |
| Method | IRC |
| Basis Set | HF/3-21G |
| Number of Points | 50 |
| Direction | Forward Only |
| Energy of Last Point | -231.61932247 a.u. |
| Gradient | 0.00000569 a.u. |
Total Energy and RMS gradient graph:
According to the graphs above, RMS gradient only had a trend of converging to zero, but not actually reach zero. Therefore, further analysis had to be done in order to give a more accurate result.
There are three approaches which have been done to get reliable results:
Method 1: run an optimization calculation of the last point on the IRC. This approach is the fastest but it is not accurate enough to reach a minimum energy and may also locate a wrong result due to the uncertainty of the endpoint.
Method 2: redo the IRC calculation with a larger number of points (in this case, 150). This approach is more reliable than method 1 but also has risk of resulting in the wrong structure due to too many points involved.
Method 3: redo IRC calculation with computing the force constants at every steps. This approach is the most reliable but is not always working for the large systems.
Method 1. Optimization of the IRC Endpoint
| Type of Transition State | Chair |
| Method | Optimization to minimum (RHF) |
| Basis Set | HF/3-21G |
| Total Energy | -231.69166702 a.u. |
| Gradient | 0.00000475 a.u. |
To access to the .log file of optimization calculation of endpoint,click here.
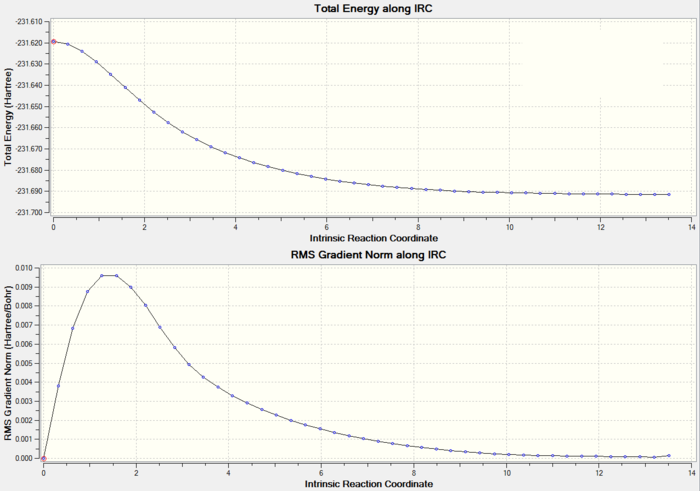
Method 2. IRC Calculation with Larger Number of Points
| Type of Transition State | Chair |
| Method | IRC |
| Basis Set | HF/3-21G |
| Number of Points | 150 |
| Direction | Forward Only |
| Energy of Last Point | -231.61932200 a.u. |
| Gradient | 0.00000581 a.u. |
Total Energy and RMS gradient graph:
To access to the .log file of IRC calculation,click here.
Re-optimization to a TS (Berny) using B3LYP/6-31G* Level of Theory
1.Key Information of Result
| Type of Transition State | Chair |
| Method | Optimization to a TS (Berny)+ Frequency Analysis |
| Basis Set | B3LYP/6-31G(d) |
| Total Energy | -234.55698303 a.u. |
| Number of Imaginary Frequency | 1 |
| Wavenumber of Imaginary Frequency | -565.54 cm-1 |
2. Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000022 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000078 0.001800 YES
RMS Displacement 0.000025 0.001200 YES
Predicted change in Energy=-3.752833D-09
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energies at 0 K (Hartrees)= -234.414929 Sum of electronic and thermal Energies at 298.15 K (Hartrees)= -234.409009
3. Vibration Mode of Imaginary Frequency
4.File Link
To access to the .log file of optimization, click here.
Second Approach: Optimization to a TS(Berny) with Frozen Coordinates
The second approach used the frozen coordinates method which set certain atoms frozen; therefore, the bond breaking and bond forming distances were set to be exactly 2.2Å.
1. Optimized Chair Transition State
Pentahelicene |
2. Key Information of Result
| Type of Transition State | Chair |
| Method | Optimization to a TS (Berny) |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Keyword | Opt=NoEigen |
| Total Energy | -234.55698246 a.u. |
| Number of Imaginary Frequency | 1 |
| Wavenumber of Imaginary Frequency | -565.63 cm-1 |
| Point Group | C2h |
3.Vibration Mode of Imaginary Frequency
4. File Link
To access to the .log file of optimization, click here
5. Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000486 0.001800 YES
RMS Displacement 0.000122 0.001200 YES
Predicted change in Energy=-8.556510D-09
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energies at 0 K (Hartrees)= -231.466700 Sum of electronic and thermal Energies at 298.15 K (Hartrees)= -231.461340
Third Approach: Optimization to a TS (Berny)with Derivative
1.Key Information of Result
| Type of Transition State | Chair |
| Method | Optimization to a TS with Derivative |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Total Energy | -231.61932238 a.u. |
| Number of Imaginary Frequency | 1 |
| Wavenumber of Imaginary Frequency | -817.90 cm-1 |
| Point Group | C2h |
2.Vibration Mode of Imaginary Frequency
3.File Link
To access to the .log file of optimization, click here.
4. Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000090 0.000450 YES
RMS Force 0.000019 0.000300 YES
Maximum Displacement 0.001483 0.001800 YES
RMS Displacement 0.000503 0.001200 YES
Predicted change in Energy=-1.010179D-07
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energiesat 0 K (Hartrees)=-231.466696 Sum of electronic and thermal Energies298.15 K (Hartrees)=-231.461337
Results and Discussion
1.Thermochemistry Results Comparison
The first approach of the chair TS was chosen to undergo the optimization using the higher basis level and the results were listed below.
| Method | Electronic Energy (a.u.) | Sum of Electronic Energy and Zero-Point Energies at 0 K (a.u.) | Sum of Electronic and Thermal Energies at 298.15 K (a.u.) | Imaginary Frequency (cm-1) | Point Group | |
| Chair TS (Normal) | TS(Berney), HF/3-21G | -231.61932247 | -231.466700 | -231.461340 | -817.93 | C2h |
| TS(Berney), B3LYP/6-31G* | -234.55698303 | -234.414929 | -234.409009 | -565.54 | C2h |
Comparing to the values on the script, all the energies matched up to an acceptable extent which indicates that the optimization was successful. It can be seen that the higher basis method gave a lower magnitude of the energies than the HF/3-21G basis; thus higher basis would provide more stable structure.
2.Geometry Comparison
| Parameter | HF/3-21G | B3LYP/6-31G* | Literature Value[2] |
| C1-C2 Distance/ Å | 2.3736 | 2.3736 | 1.599 |
| C4-C5 Distance/ Å | 2.4452 | 2.4452 | 1.899 |
| C2-C3 and C5-C6 Bond Length/ Å | 1.4075 | 1.4075 | 1.474 |
| C3-C4 and C1-C6 Bond Length/ Å | 1.4075 | 1.4075 | 1.382 |
| C4-C5-C6 Bond Angle | 119.7048o | 112.4944o | 113.4o |
| C2-C3-C4 Bond Angle | 107.4615o | 103.6352o | 106.4o |
| Dihedral Angle | 64.0448o | 65.1817o | None |
As can be seen from the table that no accurate optimization result was obtained comparing to the literature. This might be due to the inter-fragmental distance of 2.2Å which was set manually.
Optimization of the 'boat' Transition State
First Approach: Optimization from Anti 2 Conformer
First of all, optimization to TS(QTS2) was done by using the anti-conformer which had Ci symmetry as both reactant and product. As the numbering of carbon atoms would change during the reaction, the number of atoms were changed manually as shown in the following figure.
However, the optimization job failed according to the chk file, because the transition state looked like the chair transition structure. This was due to the ignorance of the possibility of central bond rotation. The calculation simply translated the top allyl fragment; therefore, the QST2 method would not be able to locate the transition state of boat structure successfully without modification of the structures of reactants and products.
Pentahelicene |
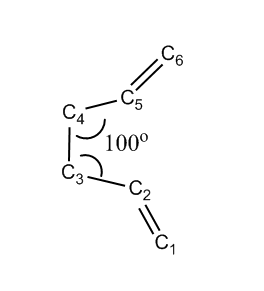
Second Approach: Optimization with Modified Reactant and Product
In order to locate the 'boat' transition state, structures of both reactants and products have been modified as shown below. The central C2-C3-C4-C5 dihedral angle was changed to be 0o and the bond angle of C2-C3-C4 as well as C3-C4-C5 were changed to be 100o.
1. Optimized Boat Transition State
Pentahelicene |
2. Key Information of Result
| Type of Transition State | Boat |
| Method | Optimization to a TS(QTS2) |
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | HF/3-21G |
| Total Energy | -231.60280200 a.u. |
| Number of Imaginary Frequency | 1 |
| Wavenumber of Imaginary Frequency | -839.94 cm-1 |
| Point Group | C2v |
3. Vibration Mode of Imaginary Frequency
4.Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000085 0.000450 YES
RMS Force 0.000029 0.000300 YES
Maximum Displacement 0.001453 0.001800 YES
RMS Displacement 0.000438 0.001200 YES
Predicted change in Energy=-4.886062D-07
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energies at 0 K (Hartrees)=-231.450928 Sum of electronic and thermal Energies at 298.15 K (Hartrees)= -231.445299
5.File Link
To access to the .log file of optimization, click here.
6. IRC Calculation
| Type of Transition State | Boat |
| Method | IRC |
| Basis Set | HF/3-21G |
| Number of Points | 50 |
| Direction | Forward Only |
| Energy of Last Point | -231.60280200 a.u. |
| Gradient | 0.00007077 a.u. |
Total Energy and RMS gradient graph:
To access to the .log file of IRC calculation, click here.
Method 1. Optimization of the IRC Endpoint
| Type of Transition State | Boat |
| Method | Optimization to minimum (RHF) |
| Basis Set | HF/3-21G |
| Total Energy | -231.68302550 a.u. |
| Gradient | 0.00000769 a.u. |
To access to the .log file of optimization calculation of endpoint,click here.
Method 2. IRC Calculation with Larger Number of Points
| Type of Transition State | Boat |
| Method | IRC |
| Basis Set | HF/3-21G |
| Number of Points | 150 |
| Direction | Forward Only |
| Energy of Last Point | -231.68393048 a.u. |
| Gradient | 0.00042262 a.u. |
Total Energy and RMS gradient graph:
To access to the .log file of IRC calculation,click here.
Method 3. Calculating Force Constant at Each Step
| Type of Transition State | Boat |
| Method | IRC |
| Basis Set | HF/3-21G |
| Number of Points | 150 |
| Direction | Forward Only |
| Energy of Last Point | -231.67591436 a.u. |
| Gradient | 0.00114584 a.u. |
Total Energy and RMS gradient graph:
Re-optimization to boat TS using B3LYP/6-31G* Level of Theory
1. Re-optimized Boat Transition State
Pentahelicene |
2. Key Information of Result
| Type of Transition State | Boat |
| Method | Optimization to a TS (QTS2) |
| Basis Set | B3LYP/6-31G* |
| Total Energy | -234.54309304 a.u. |
| Number of Imaginary Frequency | 1 |
| Wavenumber of Imaginary Frequency | -530.57 cm-1 |
3.Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000760 0.001800 YES
RMS Displacement 0.000195 0.001200 YES
Predicted change in Energy=-3.598582D-08
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energies= -234.402339 Sum of electronic and thermal Energies= -234.396005
4.Vibration Mode of Imaginary Frequency
Results and Discussion
| Method | Electronic Energy (a.u.) | Sum of Electronic Energy and Zero-Point Energies at 0 K (a.u.) | Sum of Electronic and Thermal Energies at 298.15 K (a.u.) | Imaginary Frequency (cm-1) | Point Group | |
| Boat TS | TS(QST2), HF/3-21G | -231.60280200 | -231.450928 | -231.445299 | -839.94 | C2v |
| TS(QST2), B3LYP/6-31G* | -234.54309304 | -234.402339 | -234.396005 | -530.57 | C2v |
All thermochemistry results matched the values on the script as well as the point group; thus, the analysis was successful.
Discussion
Activation Energy
Activation energy is the minimum energy required for an reaction to start. In this experiment, energies of both transition state 'chair' and 'boat' were calculated and listed in the following table.
| Type of Basis Set | HF/3-21G | HF/3-21G | B3LYP/6-31G* | Experimental | B3LYP/6-31G* |
| Temperature | at 0 K | at 298.15 K | at 0 K | at 0 K | at 298.15 K |
| Ea(Chair) /kcalmol-1 | 45.71 | 44.70 | 34.09 | 33.5 ± 0.5 | 33.19 |
| Ea(Boat) /kcalmol-1 | 55.60 | 54.72 | 42.01 | 44.7 ± 2.0 | 41.32 |
As can be seen from the table, the results from the B3LYP/6-31G* basis set was much closer to the experimental values; while the HF/3-21 basis set had about 10 kcal/mol energy difference. Thus, it would be better to use the higher basis level.
By comparing the activation energy between temperature 0K and 298.15K, it could be concluded that as temperature increases, there are more molecules which have the enough energy to come over the energy barrier. This is because as temperature increases, the average kinetic energy of the molecules are also increased; there will more molecules which have enough energy to jump over the energy barrier.
By comparing the energies between chair and boat transition state, it can be seen that chair transition state requires a lower energy than the boat state; thus, the chair transition state is more thermodynamically stable and would lead to a thermodynamic product. The boat transition state has a higher activation energy so it is kinetically stable.
To conclude, the rearrangement would likely to proceed via the chair transition state at low temperature; and via the boat transition state at high temperature.
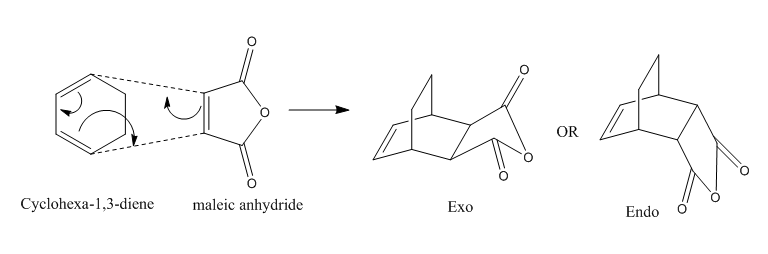
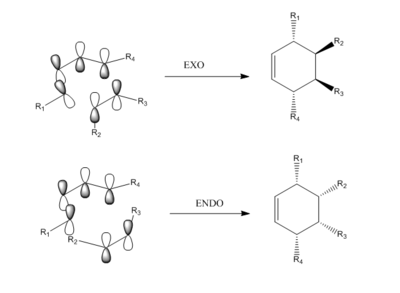
Diels Alder Cycloaddition
Introduction
Diels-Alder cycloaddition has the general mechanism as shown below. In this reaction, two new sigma bonds were formed between two terminal carbons; and also 6 pi-electrons were involved. The process is considered as concerted if the HOMO and LUMO orbitals have the same symmetry and similar energy.
Reaction between ethylene and cis-butadiene and between cyclohexa-1,3-diene and maleic anhydride were studied.
Reaction Between Ethylene and Cis-Butadiene
Optimization of Reactants
1. Optimization of Cis-butadiene
1.1 Optimized Cis-butadiene
Pentahelicene |
1.2 Key Information of Result
| Molecule | Cis-butadiene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RAM1 |
| Basis Set | ZDO (AM1 semi-empirical) |
| Total Energy | 0.04879719 a.u. |
| Charge | 0 |
| Gradient | 0.00001745 |
| Dipole Moment | 0.0414 |
| Point Group | C2v |
| Jop cpu Time | 0 days 0 hours 0 minutes 21.0 seconds |
1.3 Validity of Result
Item Value Threshold Converged?
Maximum Force 0.000030 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000408 0.001800 YES
RMS Displacement 0.000162 0.001200 YES
Predicted change in Energy=-9.691119D-09
Optimization completed.
-- Stationary point found.
1.4 File Link
To access the .log file of optimization, click here.
1.5 HOMO LUMO Molecular Orbitals
 |
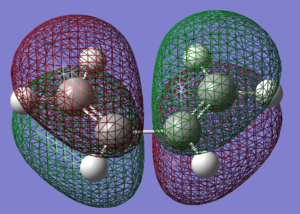
|
| LUMO | HOMO |
2. Optimization of Ethylene
1.1 Optimized Ethylene
Pentahelicene |
1.2 Key Information of Result
| Molecule | Ethylene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RAM1 |
| Basis Set | ZDO (AM1 semi-empirical) |
| Total Energy | 0.02619028 a.u. |
| Charge | 0 |
| Gradient | 0.00003647 |
| Dipole Moment | 0.0000 |
| Point Group | D2h |
| Jop cpu Time | 0 days 0 hours 0 minutes 3.0 seconds |
1.3 Validity of Result
Item Value Threshold Converged?
Maximum Force 0.000178 0.000450 YES
RMS Force 0.000053 0.000300 YES
Maximum Displacement 0.000441 0.001800 YES
RMS Displacement 0.000236 0.001200 YES
Predicted change in Energy=-5.173658D-08
Optimization completed.
-- Stationary point found.
1.4 File Link
To access the .log file of optimization, click here.
Analysis of Transition States
Optimization of Transition State using AM1 Semi-Empirical
1.Optimized Transition State
Pentahelicene |
2.Key Information of Result
| Molecule | TS |
| Method | Optimization to a minimum |
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO (AM1 semi-empirical) |
| Total Energy | 0.11165470 a.u. |
| Charge | 0 |
| Gradient | 0.00004118 |
| Dipole Moment | 0.5610 |
| Point Group | Cs |
| Number of Imaginary Frequency | 1 |
| Wavenumber of Imaginary Frequency | -957.06 cm-1 |
3.Vibration Mode of Imaginary Frequency
4. File Link
To access to the .log file of optimization, click here
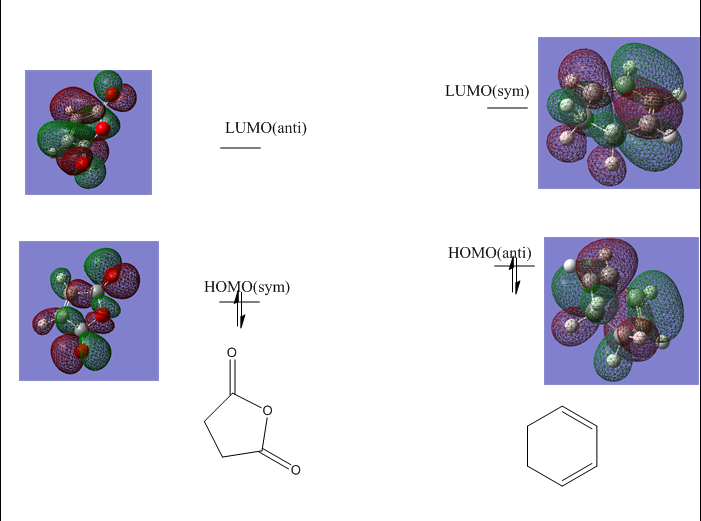
5. HOMO LUMO Molecular Orbitals
 |
|
| LUMO(Symmetric) | HOMO(Antisymmetric) |
IRC Calculation of Transition State using AM1 Semi-Empirical
Pentahelicene |
| Method | IRC |
| Basis Set | AM1 semi-empirical |
| Number of Points | 100 |
| Direction | Both directions |
| Energy of Last Point | 0.07462805 a.u. |
| Gradient | 0.00004570 a.u. |
Total Energy and RMS gradient graph:
To access to the .log file of IRC calculation,click here.
Optimization of Transition State using 6-31G(d) Basis Set
IRC Calculation of Transition State using 6-31G(d) Basis Set
| Method | IRC |
| Basis Set | 6-31G(d) |
| Number of Points | 100 |
| Direction | Both directions |
| Energy of Last Point | -234.57525785 a.u. |
| Gradient | 0.00007561 a.u. |
Results and Discussion
1.Geometry Comparison
| Terms | Result from AM1 Optimization | Result from 6-31G*Optimization | Literature Value[3] |
| C1-C2 and C3-C4 Bond Length/ Å | 1.3820 | 1.3820 | 1.383 |
| C2-C3 Bond Length/ Å | 1.3975 | 1.4072 | 1.407 |
| C5-C6 Bond Length/Å | 1.3830 | 1.3830 | 1.386 |
| C1-C6 and C4-C5 Interfragmental Distance/ Å | 2.1194 | 2.2722 | 2.273 |
The 6-31G* method provided a more accurate structure since all values were closer to literature than the AM1 semi-empirical MO method.
Reaction Between Cyclohexa-1,3-diene and Maleic Anhydride
Optimization of Reactants
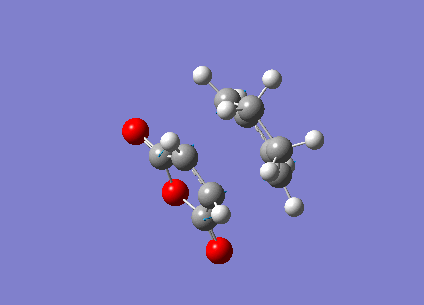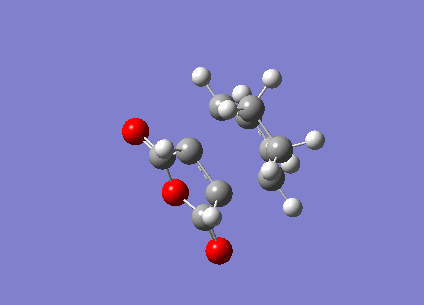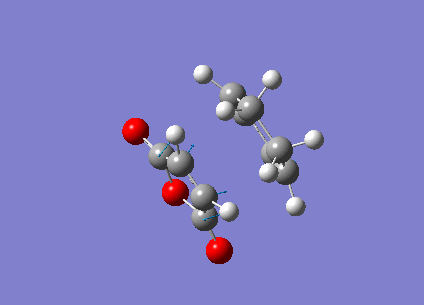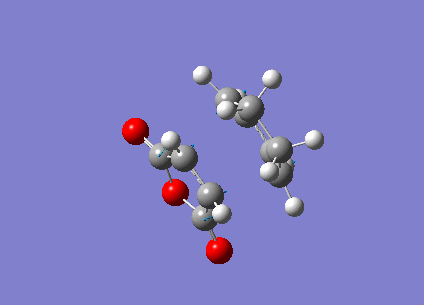
Optimization of Maleic Anhydride
1. Optimized Maleic Anhydride Molecule
Pentahelicene |
2. Key Information of Result
| Molecule | Maleic Anhydride |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Total Energy | -379.28954412 a.u. |
| Charge | 0 |
| Gradient | 0.00001745 |
| Dipole Moment | 0.0414 |
| Point Group | C2v |
| Jop cpu Time | 0 days 0 hours 1 minutes 18.0 seconds |
3. Validity of Result
Item Value Threshold Converged?
Maximum Force 0.000292 0.000450 YES
RMS Force 0.000094 0.000300 YES
Maximum Displacement 0.001428 0.001800 YES
RMS Displacement 0.000551 0.001200 YES
Predicted change in Energy=-4.590331D-07
Optimization completed.
-- Stationary point found.
4.File Link
To access the .log file of optimization, click here.
Optimization of Cyclohexa-1,3-diene
1. Optimized Cyclohexa-1,3-diene
Pentahelicene |
2. Key Information of Result
| Molecule | Cyclohexa-1,3-diene |
| Method | Optimization to a minimum |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Total Energy | -233.41893629 a.u. |
| Charge | 0 |
| Gradient | 0.00001902 |
| Dipole Moment | 0.3780 |
| Point Group | C2 |
| Jop cpu Time | 0 days 0 hours 1 minutes 18.0 seconds |
3. Validity of Result
Item Value Threshold Converged?
Maximum Force 0.000023 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000547 0.001800 YES
RMS Displacement 0.000155 0.001200 YES
Predicted change in Energy=-3.186501D-08
Optimization completed.
-- Stationary point found.
4.File Link
To access the .log file of optimization, click here.
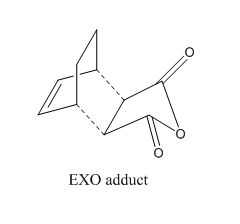
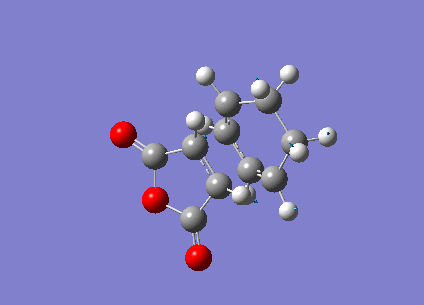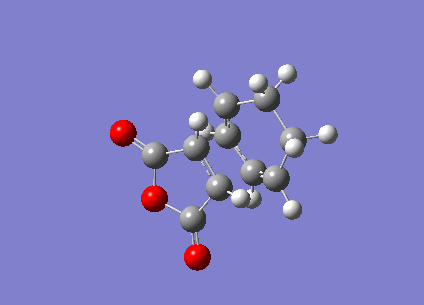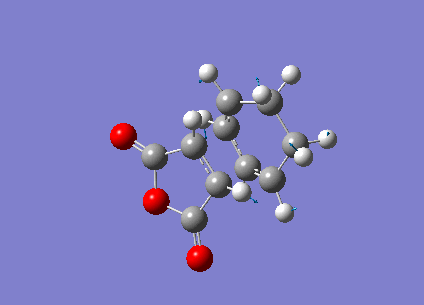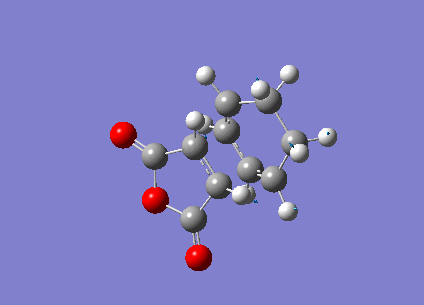
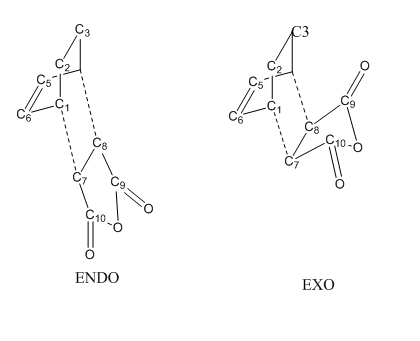
Optimization of Exo Product
1. Optimized Exo Product
Pentahelicene |
2. Key Information of Result
| Method | Optimization to TS(Berny) + Frequency |
| Basis Set | ZDO(AM1 semi-empirical) |
| Key Word | opt=noeigen |
| Total Energy | -0.05041985 a.u. |
| Number of Imaginary Frequencies | 1 |
| Wavenumber of imaginary Frequency | -812.21 cm-1 |
3.Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000023 0.001800 YES
RMS Displacement 0.000006 0.001200 YES
Predicted change in Energy=-1.390869D-11
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energies= 0.134881 Sum of electronic and thermal Energies= 0.144882
4.Vibration Mode of Imaginary Frequency
Imaginary frequency was at -812.21 cm-1.
5 Vibration Mode of First Positive Frequency
First real frequency was at 60.85 cm-1
6. File Link
To access the file link to .log file of optimization, click here
Optimization of Endo Product
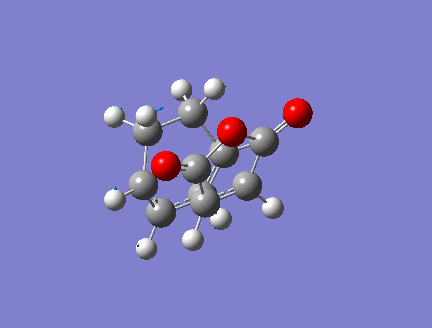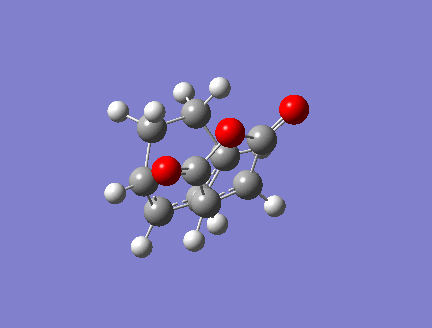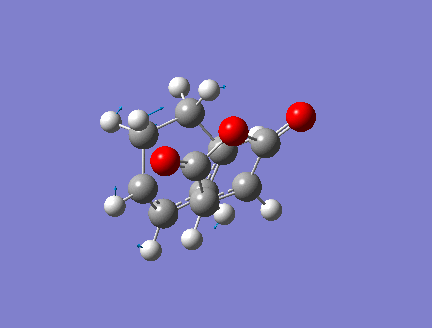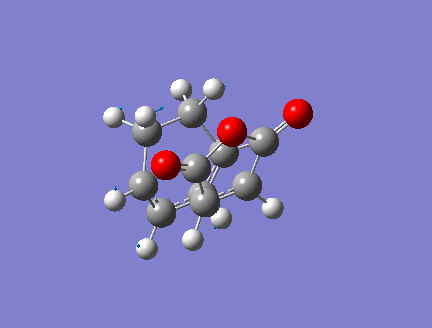
1.Optimized Endo Adduct
Pentahelicene |
2.Key Information of Result
| Method | Optimization to TS(Berny) + Frequency |
| Basis Set | ZDO(AM1 semi-empirical) |
| Key Word | opt=noeigen |
| Total Energy | -0.05150478 a.u. |
| Number of Imaginary Frequencies | 1 |
| Wavenumber of imaginary Frequency | -806.47 cm-1 |
3.Frequency Analysis
Item Value Threshold Converged?
Maximum Force 0.000031 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000999 0.001800 YES
RMS Displacement 0.000201 0.001200 YES
Predicted change in Energy=-2.291370D-08
Optimization completed.
-- Stationary point found.
Sum of electronic and zero-point Energies= 0.133494 Sum of electronic and thermal Energies= 0.143683
4.Vibration Mode of Imaginary Frequency
Imaginary frequency was at -806.47 cm-1.
5 Vibration Mode of First Positive Frequency
First real frequency was at 62.51 cm-1
6. File Link
To access the file link to .log file of optimization, click here
Result and Discussion
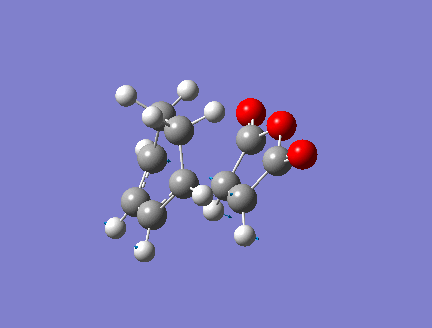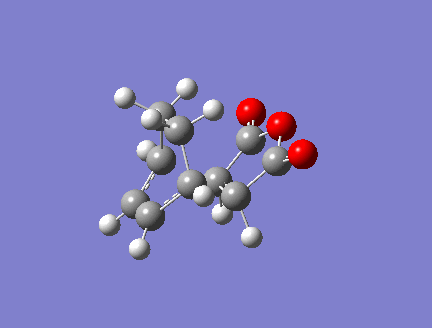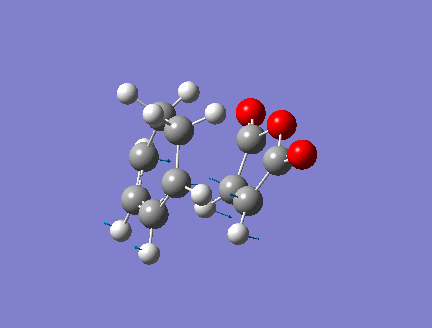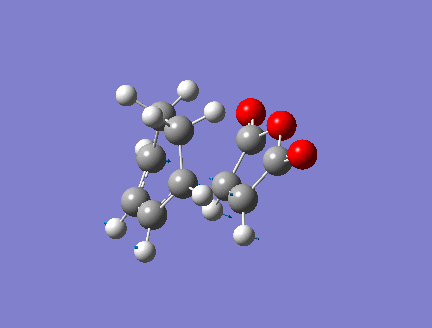
1.HOMO LUMO
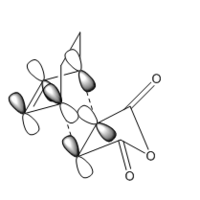
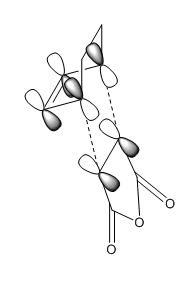
In order to have orbital overlap, the orbitals must have same symmetry and similar energy. There were two overlap interactions: symmetric overlap between HOMO of maleic anhydride and LUMO of hexadiene; and antisymmetric overlap between HOMO of hexadiene and LUMO of maleic anhydride. Details are shown in the below figure.
HOMO of EXO adduct
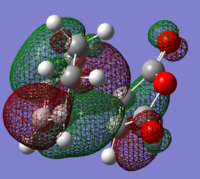

LUMO of EXO adduct
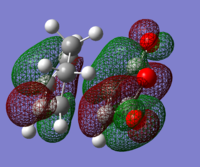
HOMO of ENDO adduct

LUMO of ENDO adduct


The energy difference between HOMO and LUMO in exo adduct is 0.30230 a.u. (189.70 kcal/mol)
The energy difference between HOMO and LUMO in endo adduct is 0.30937 a.u. (197.13 kcal/mol)
Exo adduct had quite slightly lower energy difference than endo adduct. HOMO of exo lies lower in energy than endo while LUMO of exo lies higher than endo does.
2.Geometry Comparison
| Terms | Optimized Exo TS | Optimized Endo TS |
|---|---|---|
| C5=C6 Bond Length/Å | 1.3968 | 1.3972 |
| C7-C8 Bond Length/ Å | 1.4101 | 1.4085 |
| C1-C6 and C4-C5 Bond Length/ Å | 1.3944 | 1.3930 |
| C7-C10 and C8-C9 Bond Length/ Å | 1.4882 | 1.4892 |
| C1-C7 and C4-C8 Interfragmental Distance/ Å | 2.1704 | 2.1623 |
Conclusion:
1. There was not a quite large difference in bond length between endo and exo TS.
2. Comparing to typical C-C or C=C bond length, neither of them was formed.
3. The inter-fragmental distance between C1 and C7 as well as C4 and C8 were close to 2.2Å.
3. Thermochemistry Comparison
| The total electronic energy of exo adduct | -0.05041985 a.u. |
| The total electronic energy of endo adduct | -0.05150478 a.u. |
It can be seen that there was not quite a large energy difference between the exo and endo adducts; hence, it is possible that the reaction would proceed via either of the transition states.
Secondary orbital overlap:
As can be seen from the mechanism above, the endo adduct was more sterically hindered and less stable thermodynamically, but it was the main product experimentally. This could be explained by secondary orbital overlap which states that the interaction between pi orbitals were not involved in the bond-forming reaction. Endo adduct had significant secondary orbital overlap whereas exo adduct did not. This was due to the opposite orientation between =CH-CH= and -(C=O)-(C=O)- in exo adduct. The Secondary Orbital Overlap effect overcame the steric hindrance and the endo adduct was the predominate product in this reaction.
Reference
- ↑ I. H. Gyorgy Schultz, Journal of Molecular Structure, 1994, 346, 63-69.
- ↑ Michael J. S. Dewar , George P. Ford , Michael L. McKee , Henry S. Rzepa , Leslie E. Wade. J. Am. Chem. Soc., 1977, 99 (15),5069–5073
- ↑ Goldstein, E.; Beno, B.; Houk, K. N.; J. Am. Chem. Soc., 1996, 118, 6036-6043. DOI:10.1021/ja9601494