Rep:Mod:yhw14cts
Transition States and Reactivity
Yi Hang Cherie WONG (yhw14) CID: 00933828
Introduction
A potential energy surface is a mathematical or graphical function that gives relationship between the energy of a molecule and its geometry with the relative positions of the atoms participating in the reaction. The stationary points may be classified according to the first and second derivatives of the energy with respect to position. At the minimum of a reaction profile, the gradient is represented by the first derivative of the reaction coordinate, which is zero, while curvature is represented by the second derivative, is positive in this case; hence energy rises in all directions. Energy minima correspond to physically stable chemical species, which could be reactants and products. The transition state is defined as the maximum in a reaction profile, where the gradient is again, zero, while curvature is negative; hence energy decreases in one direction, which indicates the reaction pathway of the chemical reaction. The potential energy surface can be computed using GaussView, which is a graphical interface for Gaussian, where structure and energy of reactants or products can be modelled to illustrate the transition states, which can rarely be obtained experimentally. The intrinsic reaction coordinate can then be calculated and compared to predict the reaction path at a transition state and follow it to the correct minima.
In this computational lab, all of the reactants and products were optimised to their minima, and the transition states were also optimised. The calculations were done by GaussView, using mainly semi-empirical method PM6 and Density Functional Theory-B3LYP-631G. The latter is a more detailed and accurate optimisation, which is more time consuming as it involves a higher number of basis set. Frequency calculations were performed to show molecular vibrations to confirm the position on the potential energy surface. If all the vibrational frequencies are real, this confirms the structure is a minimum, and vice versa, the presence of imaginary frequency may suggest that the structure is at its transition state. Intrinsic reaction coordinate method was carried out using calculated force constants to predict which conformer a reaction path from the transition state would lead to.
Nf710 (talk) 12:15, 24 February 2017 (UTC) Nice intro, TS well defined could have gone into more detail about the methods
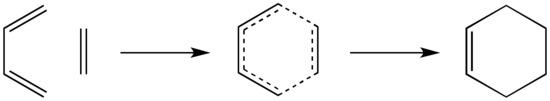
Exercise 1: Reaction of Butadiene with Ethene
The reaction between butadiene and ethene is a typical pericyclic [4+2] Diels-Alder reaction that proceeds via a concerted mechanism through a cyclic transition state. The reaction scheme is shown below.
Molecular Orbital Analysis
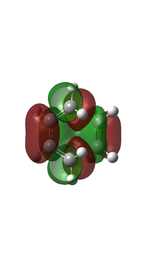
The diagram below illustrates the π molecular orbitals involved in the formation of the transition state between the HOMO and LUMO of butadiene and ethene.

(Fv611 (talk) 11:54, 24 February 2017 (UTC) Good diagram, but the TS MOs do not have the symmetry labels.)
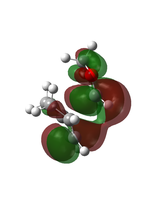
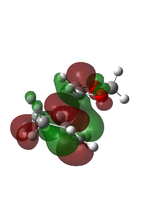
Butadiene and ethene were optimised to their minima at semi-empirical method PM6 level. The computed π MOs were shown in the following table.
(Fv611 (talk) 11:54, 24 February 2017 (UTC) These types of pictures make it very hard to see whether you have the right MOs or not.)
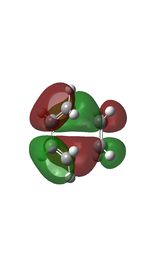
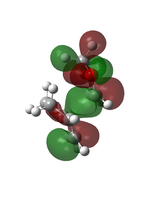
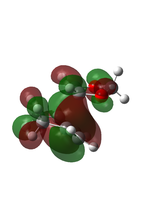
The transition state was first optimised to its minimum, followed by a transition state calculation at semi-empirical method PM6 level. The MOs computed were shown below.
Based on the MO diagram and the computed MOs shown above, a reaction is only allowed when the MOs with the same symmetry interact with each other, i.e. symmetric-symmetric and asymmetric-asymmetric interactions; and a reaction is forbidden when the MOs with different symmetry interact with each other, i.e. symmetric-asymmetric interactions. The MOs have to be close in energy in order to overlap effectively.
In the reaction between butadiene and ethene, the butadiene asymmetric MO interacts with the ethene asymmetric MO to give the MOs 2 and 5 of the transition state, and the butadiene symmetric MO interacts with the ethene symmetric MO to give the MOs 3 and 4 of the transition state. Therefore, a reaction is allowed when the symmetry labels of the MOs of the reactants are the same; and forbidden when the symmetry labels are different.
The orbital overlap integral is zero in a symmetric-asymmetric interaction and non-zero in symmetric-symmetric and asymmetric-asymmetric interactions.
Bond Length Analysis
The changes in bond lengths between carbons in reactant, transition state and product as the reaction progresses were studied by comparing the bond lengths between carbons before and after the reaction. The summary of bond lengths is shown below.
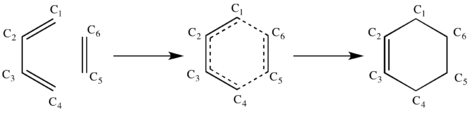
| State | C1-C2 | C2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-C1 |
|---|---|---|---|---|---|---|
| Reactants | 1.335 | 1.468 | 1.335 | / | 1.327 | / |
| Transition State | 1.380 | 1.411 | 1.380 | 2.115 | 1.382 | 2.114 |
| Product | 1.500 | 1.338 | 1.500 | 1.540 | 1.540 | 1.540 |
| sp3-sp3 (C-C single bond) [1] | sp2-sp2 (C-C double bond) [1] | Van der Waals radius of the C atom [2] | |
|---|---|---|---|
| Bond Lengths (Å) | 1.54 | 1.33 | 1.7 |
As the reaction progresses, the bond lengths between C1-C2, C3-C4 and C5-C6 lengthen from ~1.3 Å to ~1.5 Å as the bond order decrease to one, whereas bond length of C2-C3 shortens from ~1.5 Å to ~1.3 Å as the bond order increases from one to two. This could be explained as the increase in bond length suggest a change from sp2-sp2 (C-C double bond) with typical bond length of 1.33 Å [1] to sp3-sp3 (C-C single bond) with typical bond length of 1.54 Å [1], and vice versa for the decrease in bond length. Based on the calculated bond lengths above in Table 1, it is clear that bond lengths and bond orders of the reactants changed during the reaction and the new bonds formed at C4-C5 and C6-C1 are single bonds as they have bond lengths of 1.54 Å. The typical Van der Waals radius of the C atom is 1.7 Å [2]. As the bond lengths between C4-C5 and C6-C1 of the transition state are shorter than 2 x Van der Waals radii of C, this reflects that C-C bonds are forming at the transition state.
Vibration Analysis
The imaginary frequency at -948.32 cm-1 corresponds to the reaction path at the transition state, which shows a synchronous bond formation, which agrees with the concerted mechanism of [4+2] cycloaddition, where both reaction centres converge at the same time to form two new bonds.
| Reaction Path at the Transition State |
The lowest positive frequency at 145.14 cm-1 is asynchronous as shown below, where one of the C from the ethene moves towards the butadiene reaction centre and the other C moves away. This suggests that bonds are formed one at a time.
| Lowest positive frequency |
(Fv611 (talk) 11:54, 24 February 2017 (UTC) You have the right TS, but the way you presented both frequencies saying first that bond formation is synchronous and then asynchronous makes me think that you don't understand which vibration tells us about the bond-formation step.)
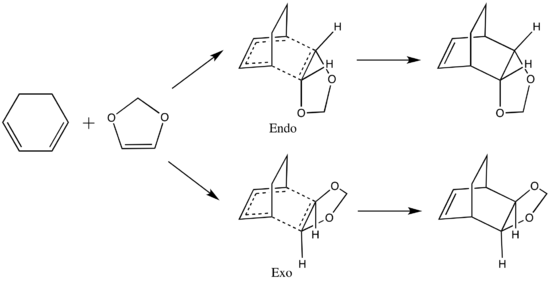
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
The Diels-Alder reaction between cyclohexadiene and 1,3-dioxole can proceed via two different pathways, endo and exo, which are shown in the reaction scheme below. GaussView calculations could be carried out to compare the reaction barriers and reaction energies of the two pathways to determine the kinetic and thermodynamic products.
(Gaussian is the calculation software, GaussView is simply a visualiser/graphical user interface Tam10 (talk) 17:14, 20 February 2017 (UTC))
Frequency Analysis
Frequency calculations were run to confirm that reactants (cyclohexadiene and 1,3-dioxole), and both endo and exo products did not have imaginary vibrations, suggesting they are structures at relative minima to the transition state; whereas both endo and exo transition states each had one imaginary vibration.
Molecular Orbital Analysis
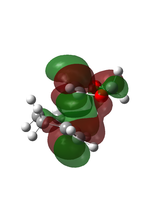
Cyclohexadiene and 1,3-dioxole were optimised to their minima at DFT-B3LYP 631-G level. The computed π MOs were shown in the following table.
| Molecule | Cyclohexadiene | 1,3-Dioxole |
|---|---|---|
| LUMO |  |
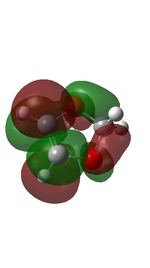 |
| Corresponding to the s orbital | Corresponding to the a orbital | |
| HOMO | 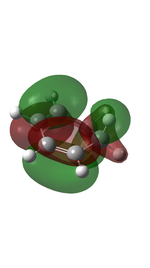 |
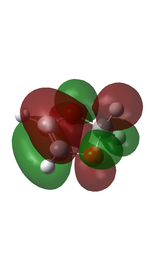 |
| Corresponding to the a orbital | Corresponding to the s orbital |
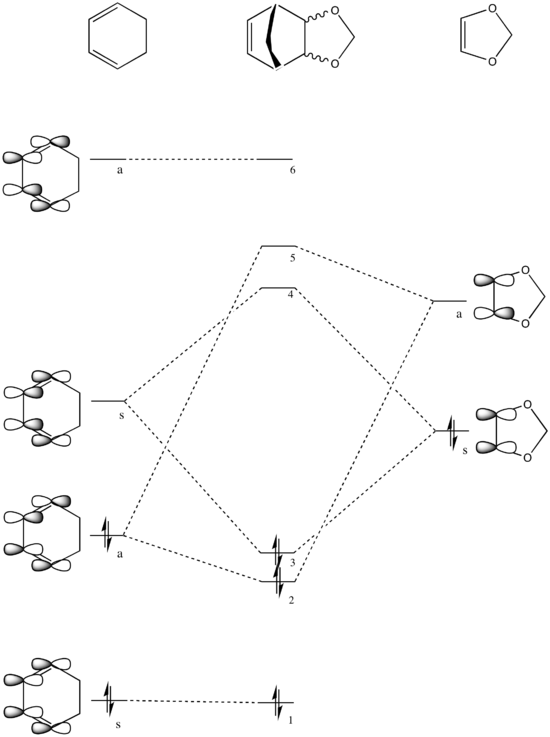
The transition state was first optimised to its minimum, followed by a transition state calculation at DFT-B3LYP 631-G level. The MOs computed were shown below.
The diagram below illustrates the π molecular orbitals of cyclohexadiene and 1,3-dioxole involved in the formation of the transition state.
Normal Demand vs Inverse Demand Diels-Alder Reaction
A normal electron demand Diels-Alder reaction can be defined as a reaction between electron rich diene and electron poor dienophile. An inverse electron demand is the reaction between electron poor diene and electron rich dienophile.
The LUMO of cyclohexadiene and the HOMO of 1,3-dioxole are very close in energy, which results in strong bonding interaction between the two to form the HOMO and LUMO of both endo and exo TS. Since the LUMO of cyclohexadiene and the HOMO of 1,3-dioxole have the correct symmetry (symmetric) to interact, they interact to form the symmetric HOMO and LUMO of both TS.
The presence of the two electron donating oxygen atoms on 1,3-dioxole raise the energy of both its HOMO and LUMO, making the overlap between the LUMO of cyclohexadiene and the HOMO of 1,3-dioxole much better than the HOMO of cyclohexadiene and the LUMO of 1,3-dioxole. Thus, the LUMO of cyclohexadiene and the HOMO of 1,3-dioxole are now the frontier orbitals that interact the most, hence this is an inverse demand Diels-Alder reaction.
Reaction Energies and Secondary Orbital Interaction
The reaction paths at the endo and exo transition states are shown below.
| Reaction Path at the Endo Transition State | Reaction Path at the Exo Transition State | ||||
|---|---|---|---|---|---|
The energies of reactants were taken using the sum of the energies of cyclohexadiene and 1,3-dioxole optimised to their minima at DFT-B3LYP 631-G. The calculations were done using DFT-B3LYP 631-G.
| Reactants | Transition State | Product | Activation Energy | Gibbs Free Energy | |
|---|---|---|---|---|---|
| Endo | -1313782 | -1313622 | -1313849 | 160 | -67 |
| Exo | -1313782 | -1313614 | -1313846 | 168 | -64 |
Kinetically favourable product of a reaction is the one that has the lowest activation energy; Thermodynamically favourable product is the product with lowest energy conformer, hence the more stabilised product.
The endo transition state is the kinetic product of this reaction as it has lower activation energy. This could be explained using the secondary orbital interaction between the lone pair orbital on the oxygen atom and the empty π* orbital of diene. This favourable effect stabilises the endo transition state.
As the oxygen atom lone pair is oriented away from the diene π system in the exo transition state, the secondary orbital effect is not present, hence the activation energy of exo reaction is higher.
However, the exo transition state is thermodynamically favourable due to less steric hindrance, hence if sufficient energy is supplied to the system, i.e. increasing temperature, formation of the exo product would be more favourable.
Nf710 (talk) 12:20, 24 February 2017 (UTC) You got the correct results! but you have said them wrong in your conclusions in this system the exo has more steric hinderence!
Nf710 (talk) 12:23, 24 February 2017 (UTC) It would have been nice if you could have shown the SOO in a diagram
Exercise 3: Diels-Alder vs Cheletropic
Xylylene can react with sulfur dioxide through Diels-Alder reaction via either endo or exo pathways to form a 6-membered ring, or through cheletropic reaction to form a 5-membered ring. The reaction scheme is shown below. Reaction barriers and reaction energies for each pathway were compared to determine the reaction that is most favourable.

Intrinsic Reaction Coordinate
The following files show the intrinsic reaction coordinates of the three different reaction pathways of xylylene and SO2. Please click to see animation.
| IRC of Diels Alder Reaction via Endo TS | IRC of Diels Alder Reaction via Exo TS | IRC of Cheletropic TS |
|---|---|---|
 |
 |
 |
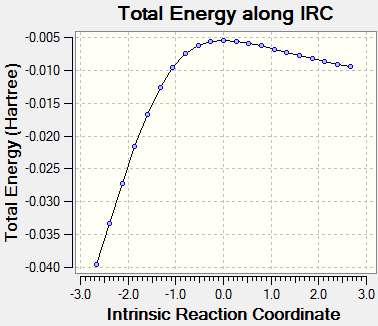 |
 |
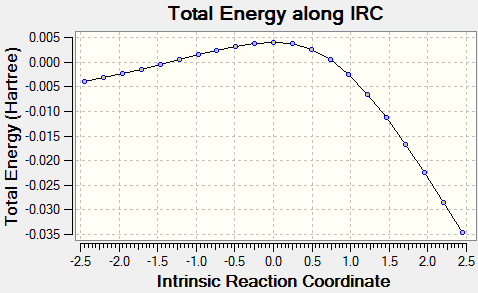 |
(IRCs are incomplete. You need to increase the number of steps until convergence is reached Tam10 (talk) 17:14, 20 February 2017 (UTC))
Reaction Energies and Reaction Barriers
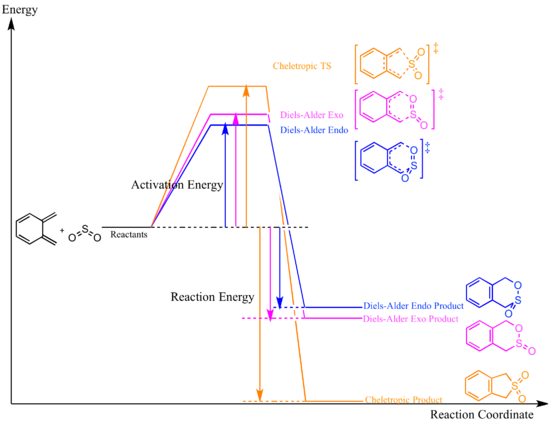
The energies of reactants were taken using the sum of the energies of xylylene and SO2 optimised to their minima. All calculations were done using semi-empirical PM6 method.
| Reactants | Transition State | Product | Activation Energy | Reaction Energy | |
|---|---|---|---|---|---|
| Endo | 154.376786 | 237.767824 | 56.9576013 | 83.391038 | -97.4191847 |
| Exo | 154.376786 | 241.753433 | 56.3196048 | 87.406647 | -98.0571812 |
| Cheletropic | 154.376786 | 260.08205 | 0.013127501 | 105.705264 | -154.3636585 |
With reference to the reaction profile and Table 4, the Diels-Alder reaction via the endo pathway has the lowest activation energy, making it a more kinetically favourable product, i.e. the kinetic product; the Diels-Alder exo product has a lower energy than endo, however with a higher activation energy hence it will not form under low temperature condition; the cheletropic product is the most stabilised product out of the three and it has the lowest reaction energy, therefore is the thermodynamic product.
Instability of Xylylene
Xylylene does not follow the Hückel rule (4n+2 electrons) hence it is not aromatic. In addition, it is rich in double bonds, all these factors make xylylene a very unstable molecule.
Electrocyclic reaction to form benzocyclobutane could also be another possible reaction pathway apart from Diels-Alder reaction and cheletropic reaction. During all the reactions including the Diels-Alder and cheletropic reactions, as well as the electrocyclic reaction, they aromatise the 6-membered ring and the driving force of these reactions is the formation of the aromatic benzene ring, which stabilise the reaction to become more thermodynamically favourable.
Side Reaction between cis-diene in Xylylene 6-membered ring and SO2
o-Xylylene contains another cis-butadiene fragment located in the 6-membered ring that can undergo a Diels-Alder reaction with SO2. The reaction energies and reaction barriers between endo and exo pathways are compared in the table below.
The energies of reactants were taken using the sum of the energies of xylylene and SO2 optimised to their minima. All calculations were done using semi-empirical PM6 method.
| Reactants | Transition State | Product | Activation Energy | Reaction Energy | |
|---|---|---|---|---|---|
| Endo | 154.376786 | 267.984805 | 172.272196 | 113.608019 | 17.89541 |
| Exo | 154.376786 | 275.821924 | 176.711916 | 121.445138 | 22.33513 |
It is clear that both endo and exo Diels-Alder side reactions are kinetically and thermodynamically unfavourable at this site as the activation energies are much higher than the previous reactions mentioned above in Table 4 and the reaction energies show that the reactions are endothermic.
(Very well answered and complete section Tam10 (talk) 17:14, 20 February 2017 (UTC))
Conclusion
The transition states of three pericyclic reactions were investigated in this computational lab, using GaussView to optimise the reactants, transition states and products respectively, and the vibrational frequencies and intrinsic reaction coordinates were computed.
In the reaction between butadiene and ethene, the importance of molecular orbital symmetry in the interaction of molecular orbitals was illustrated, such that a reaction is only allowed when the molecular orbitals with the same symmetry interact with each other and forbidden when symmetric molecular orbital interact with asymmetric orbitals. Furthermore, the vibration frequency calculation proved that this [4+2] Diels-Alder reaction proceeds via a concerted mechanism as synchronous bond formation was demonstrated. Bonding interaction can be further confirmed as the bond distance between the two termini carbon atoms in the transition state is shorter than the sum of two Van der Waals radii of carbon.
In the reaction of cyclohexadiene and 1,3-dioxole, both the endo and exo transition states were investigated. In general, the endo transition state is kinetically more favourable as it has lower activation barrier, possibly due to the secondary orbital interaction between the lone pair in p orbital on the oxygen atom and the empty π* orbital in the diene, which stabilises the transition state. However, the exo transition state is thermodynamically favourable due to less steric hindrance, hence if sufficient energy is supplied to the system, formation of the exo product could be possible.
The final reaction between xylylene and SO2 again proved that the endo Diels-Alder transition state has the lowest activation energy, making it kinetically favourable at low temperature. However, the cheletropic product is the most stabilised and thermodynamically favourable product. Therefore the reaction would yield the cheletropic product under thermodynamic control instead of the exo Diels-Alder product.
References
- ↑ 1.0 1.1 1.2 1.3 L. Pauling and L. O. Brockway, Journal of the American Chemical Society, 1937, Volume 59, Issue 7, pp. 1223-1236, DOI: 10.1021/ja01286a021, http://pubs.acs.org/doi/abs/10.1021/ja01286a021
- ↑ 2.0 2.1 S. S. Batsanov, Inorganic Materials, 2001, Volume 37, Number 9, pp. 871-885, https://physlab.lums.edu.pk/images/f/f6/Franck_ref2.pdf.