Rep:Mod:yht17
Part 1
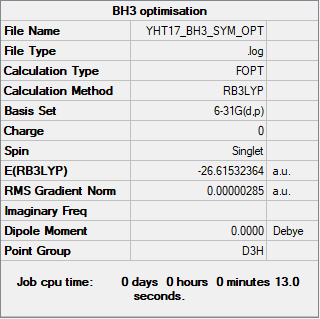
BH3
Method: RB3LYP
Basis set: 6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000023 0.001800 YES RMS Displacement 0.000015 0.001200 YES
Frequency analysis log file: YHT17_BH3_FREQ.LOG
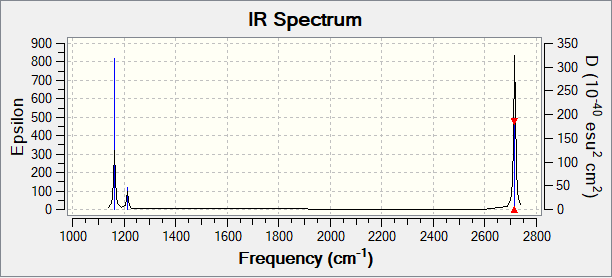
Low frequencies --- -2.2126 -1.0751 -0.0055 2.2359 10.2633 10.3194 Low frequencies --- 1162.9860 1213.1757 1213.1784
Optimised BH3 molecule |
| Vibration | Frequency (cm-1) | Intensity (au) | Symmetry | IR active? | Type |
|---|---|---|---|---|---|
| 1 | 1163 | 93 | a2" | Yes | Out-of-plane bend |
| 2 | 1213 | 14 | e' | Very slight | In-plane bend |
| 3 | 1213 | 14 | e' | Very slight | In-plane bend |
| 4 | 2582 | 0 | a1' | No | Symmetric stretch |
| 5 | 2715 | 126 | e' | Yes | Asymmetric stretch |
| 6 | 2715 | 126 | e' | Yes | Asymmetric stretch |
There are 6 vibration modes, but only three peaks can be seen on the spectrum. For a vibration mode to be IR active, it must involve a change in dipole moment. As mode 4 is totally symmetric, it does not involve a change in dipole moment and therefore does not appear on the IR spectrum. Out of the 5 vibration modes that do involve a change in dipole moment, there are two degenerate pairs. Modes 2 and 3 have the same frequency, as do modes 5 and 6. These degenerate pairs overlap on the spectrum and appear as 1 peak each. As a result, there are only 3 visible peaks on the spectrum.
Good explanation with both reasons given, the detail of the bend types in the vibrational analysis table is also good. Smf115 (talk) 21:27, 1 June 2019 (BST)
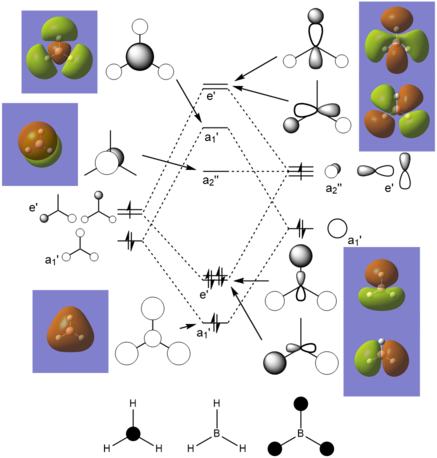
MO Diagram:
MO theory predicts the combinations of the atomic orbitals well, as well as any degenerate MOs. It can predict the approximate shapes of the MOs, but there is quite a large deviation from the actual shapes, so it cannot predict the effects of the orbital shapes on reactivity very well.
Clear inclusion of the calculated MOs with the LCAO MOs on to the diagram. Your comparison of the two is ok but very brief and general, it would have been good to see you consider some of the specific differences by looking at the 2e' or 3a1' orbitals for example. Smf115 (talk) 21:30, 1 June 2019 (BST)
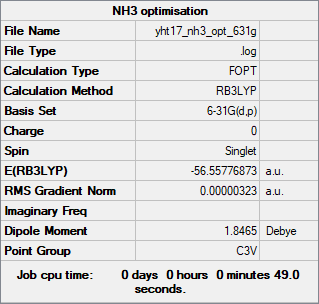
NH3
Method: RB3LYP
Basis set: 6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES
Frequency analysis log file: YHT17_NH3_FREQ.LOG
Low frequencies --- -8.4776 -8.4300 -0.0027 0.0337 0.1929 26.4250 Low frequencies --- 1089.7611 1694.1863 1694.1866
Optimised NH3 molecule |
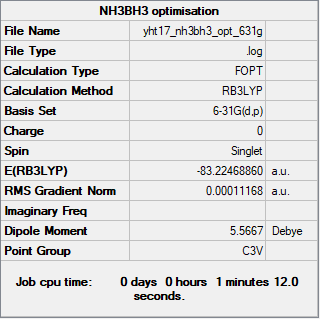
NH3BH3
Method: RB3LYP
Basis set: 6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000233 0.000450 YES RMS Force 0.000083 0.000300 YES Maximum Displacement 0.001201 0.001800 YES RMS Displacement 0.000369 0.001200 YES
Frequency analysis log file: YHT17_NH3BH3_FREQ.LOG
Low frequencies --- -0.0757 -0.0463 -0.0111 18.3546 18.5273 44.8329 Low frequencies --- 266.2035 634.6082 640.2843
Optimised NH3BH3 molecule |
NH3-BH3 Association Energy
E(BH3) = -26.6153 au
E(NH3) = -56.5577 au
E(NH3BH3) = -83.2246 au
ΔE = -0.0516 au = -135 kJ mol-1
Compared to most other single bonds, this is quite a weak bond.
Your calculated value is correct but you haven't shown the calculation at all. The bond strength should have also been evaluated by comparing against some relevant literature bond dissociation energies. Smf115 (talk) 21:34, 1 June 2019 (BST)
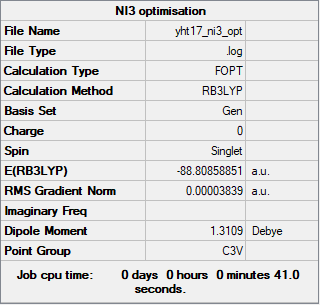
NI3
Method: RB3LYP
Basis set: N: 6-31G(d,p), I: LanL2DZ
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000493 0.001800 YES RMS Displacement 0.000333 0.001200 YES
Frequency analysis log file: Yht17_ni3_freq.log
Due to an error, the log file could not be published to D-space, so instead it has been uploaded to this wiki.
Low frequencies --- -12.7380 -12.7319 -6.2907 -0.0040 0.0188 0.0633 Low frequencies --- 101.0326 101.0333 147.4124
Optimised NI3 molecule |
The optimised N-I bond length is 2.184 Å.
Part 2 - Project: Ionic Liquids
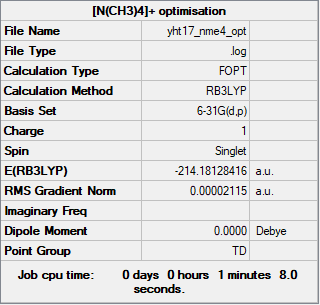
[N(CH3)4]+
Method: RB3LYP
Basis set: 6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000252 0.001800 YES RMS Displacement 0.000081 0.001200 YES
Frequency analysis log file: YHT17_NME4_FREQ.LOG
Low frequencies --- 0.0009 0.0013 0.0013 35.2977 35.2977 35.2977 Low frequencies --- 217.4079 316.4871 316.4871
Optimised [N(CH3)4]+ ion |
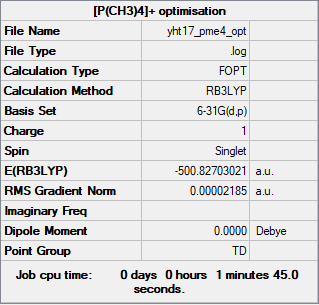
[P(CH3)4]+
Method: RB3LYP
Basis set: 6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000666 0.001800 YES RMS Displacement 0.000277 0.001200 YES
Frequency analysis log file: YHT17_PME4_FREQ.LOG
Low frequencies --- -0.0031 -0.0028 -0.0001 51.2698 51.2698 51.2698 Low frequencies --- 186.5950 211.3904 211.3904
Optimised [P(CH3)4]+ ion |
Charge Distributions
| Ion | Image | Charge on central atom (N/P) | Charge per C atom | Charge per H atom |
|---|---|---|---|---|
| [N(CH3)4]+ | 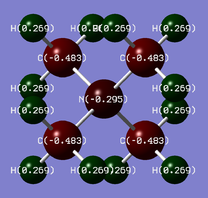 |
N: -0.30 | C: -0.48 | H: +0.27 |
| [P(CH3)4]+ | 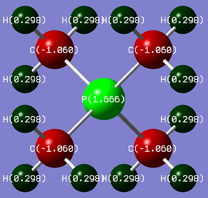 |
P: +1.67 | C: -1.06 | H: +0.30 |
The charge spread is quite different between the two ions. In the nitrogen ion, N is the most electronegative element, so the N atom has a negative charge. However, the C atoms have more negative charge because they are each surrounded by 3 H atoms, which is the most electropositive element. The positive charge is all spread out over the H atoms. This is in contrast to the location of the charge in a traditional depiction, where the positive charge would be on the N atom. This is because the traditional depiction does not take into account the electronegativities of atoms, it assumes electron density is evenly distributed in a bond.
In the phosphorus ion, P is more electropositive than C and about the same as H. Thus, the P atom does have a positive charge, and in fact has more positive charge than in a traditional depiction.
In both ions, the H atoms have a similar charge, suggesting that the inductive effect from the central atom has minimal effect.
Correct NBO charges calculated and good use of a uniform charge distribution across both ILs. Your discussion using the relative electronegativities is ok but could be improved by including electronegativity values, for example, and could be a bit more developed. Smf115 (talk) 18:57, 4 June 2019 (BST)
Nice mention that the traditional picture doesn't account for electronegativity, however, you haven't explained why the +1 formal charge on the N arises (consider formal electron counting/Lewis structures). Smf115 (talk) 18:57, 4 June 2019 (BST)
[N(CH3)4]+ Molecular Orbitals
| MO number | Energy (au) | JSmol image | Still image | LCAO diagram | Bonding character | ||
|---|---|---|---|---|---|---|---|
| 21 (HOMO) | -0.5793 |
|
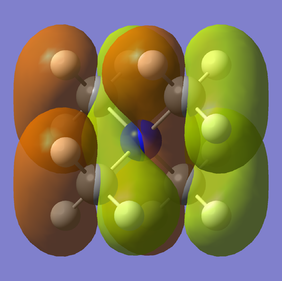 |
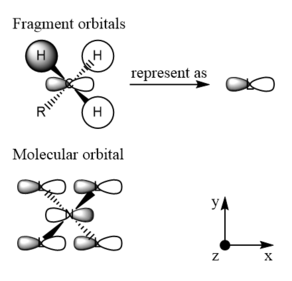 |
Weakly bonding | ||
| 16 | -0.5804 |
|
 |
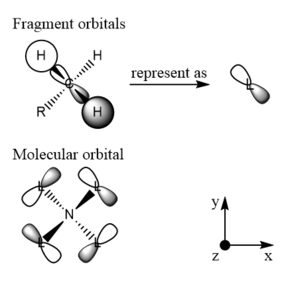 |
Non-bonding | ||
| 12 | -0.6989 |
|
 |
 |
Strongly bonding |
Very nicely presented with good use of jmols. You've chosen a good range of MOs which is great to see and your final evaluations of the MO character are good. The FOs for MO12 and 16 are not correct though and it would have helped to apply the BH3 MO diagram to CH3 to identify the correct ones. To improve, it would have also been nice to see some analysis to justify the evaluated MO character. Smf115 (talk) 19:07, 4 June 2019 (BST)
Overall, a good report which lacked further analysis in the project section. Smf115 (talk) 19:07, 4 June 2019 (BST)