Rep:Mod:yh3511
Computational Physical Chemistry
In this set of computational experiments, the transition structures of the Cope rearrangement and Diels-Alder cycloadditions are analysed using Gaussian. Bond lengths and corresponding energies of each transition state are calculated. These information are used to understand the reaction path of the reaction.
The Cope Rearrangement
1,5-hexadiene, optimized using HF/3-21G
| Energy/Hartrees | Point group | Image | |
| Anti 1 | -231.69260236 | C2 | 
|
| Anti 2 | -231.69253530 | Ci | 
|
| Anti 4 | -231.696097055 | C1 | 
|
| Gauche 1 | -231.68771615 | C2 | 
|
| Gauche 4 | -231.69153036 | C2 | 
|
Comparing a.p.p confirmations with gauche ones, it is observed that generally gauche structures have a lower energy than a.p.p ones. Therefore the lowest energy confirmation of 1,5-hexadiene may be one of the gauche confirmations (Gauche 1).
1,5-hexadiene (anti 2), optimized using B3LYP/6-31G*
Energy: -234.61171035 ( Energy is significantly smaller compared to the one run by HF/3-21G)
The structure looks similar with the one calculated before.
Sum of electronic and zero point energies: -234.469204
Sum of electronic and thermal energies: -234.461857
Sum of electronic and thermal enthalpies: -234.460913
Sum of electronic and thermal free energies: -234.50077
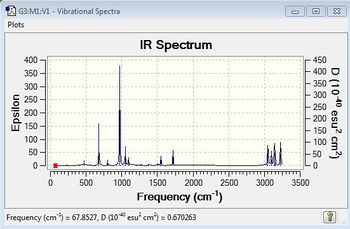
1,5-hexadiene (anti 2), optimized frequency
No negative frequency observed.
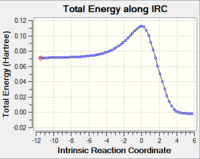
Optimization of the "Chair" and "Boat" Transition Structures
Optimized CH2CHCH2 using HF/3-21G
By copying the image into a new Gaussian window and setting the distance between the two terminal carbons as 2.2Å, a guess strucure of the chair is generated.
Hartree Fock and the default basis set 3-21G is used for the following parts in this section.
Chair structure
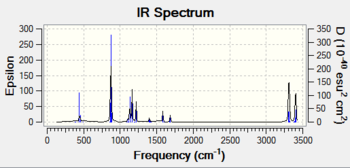
IR spectrum from TS Berny (no imaginary frequency)
Boat structure
Animation
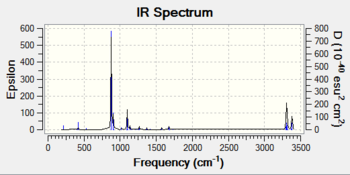
IR spectrum (one negative frequency at 840.00 cm-1)
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| Boat TS | -234.54309307 | -234.402342 | -234.3960006 |
| Chair | -234.55698303 | -234.414929 | -234.409009 |
| Anti 2 | -234.61171035 | -234.469204 | -234.461857 |
| Electronic energy | Sum of electronic and zero-point energies | Sum of electronic and thermal energies | |
| Boat TS | -231.60280161 | -231.450927 | -231.445298 |
| Chair | -231.61932237 | -231.466700 | -231.461341 |
| Anti 2 | -231.692538 | -231.539535 | -231.532564 |
| B3LYP/6-31G* | HF-3-21G | |||
| at 0 K | at 298.15 K | at 0 K | at 298 K | |
| Boat | 41.96 | 41.33 | 55.60 | 54.76 |
| Chair | 34.06 | 33.16 | 45.70 | 44.69 |
The Diels Alder Cycloaddition
AM1 semi-empirical molecular orbital method was used for the following calculations.
i) cis butadiene
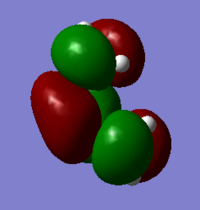
HOMO
Antisymmetric

LUMO
Symmetric
ii)Transition State geometry
QTS2 method was used
Transition state
New bond length:2.11956Å and 2.11857Å
Energy: 0.112a.u.
Lowest positive frequency:147.19cm-1
HOMO

Antisymmetric
LUMO

Symmetric
The animation of the transition state
The animation of the lowest frequency
Discussion
Typical sp3 bond length is 1.54 Å and typical sp2 bond length is 1.34 Å.[1]
The van der Waals radius of the C atom is 1.7Å.[2]
The newly formed C-C bonds are longer than typical sp2 and sp3 c-c bonds, but shorter than two vdW radius of carbon atoms. This suggests there are some interaction between the two carbons, but the distance is not close enough to form a bond. This is the evidence of a transition state structure. Also the two new C-C bonds are about the same in length, which means bond formation is synchronous. This is confirmed by the vibration at the imaginary frequency. The vibration of the lowest positive frequency however only represents the vibration of the molecule instead of the transition state.
For the symmetric LUMO, from the MO diagram it is clear that there are no overlap between the ethylene and butadiene orbitals. The overlap only exits in the antisymmetric HOMO. Therefore the HOMO from butadiene and the LUMO from ethene are used to form this transition state antisymmetric MO. This reaction is symmetry allowed.
iii)Diels Alder Reaction
Endo transition state
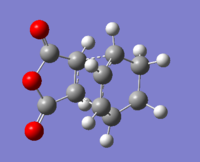
Energy: -0.05150464 a.u. Sum of electronic and zero-point Energies=0.133494 a.u. Sum of electronic and thermal Energies=0.143683 a.u.
New bond length:2.1616Å and 2.1628Å (longer than normal C-C bonds
C-C through space distances between the -(C=O)-O-(C=O)- fragment: 2.2792Å for both
C-C through space distances between C atoms of the “opposite” -CH=CH: 2.8912Å and 2.8905Å
C-C bondg lengths are shorter in the maleic anhydride ring than in the Cyclohexa-1,3-diene ring
HOMO
Antisymmetric
Exo transition state
Energy: -0.05041976 a.u. Sum of electronic and zero-point Energies=0.134881 a.u. Sum of electronic and thermal Energies=0.144882 a.u.
New bond length:2.171Å and 2.170Å (longer than normal C-C bonds)
C-C through space distances between the -(C=O)-O-(C=O)- fragment: 2.2796Å
C-C through space distances between C atoms of the “opposite” -CH=CH: 2.9451Å and 2.9450Å
C-C bond lengths are shorter in the maleic anhydride ring than in the Cyclohexa-1,3-diene ring, the same as endo
HOMO

Antisymmetric
Discussion
In the structure of the endo form, there is a favourable overlap between the π orbitals on the maleic anhydride and the diene HOMO. The exo product can be considered to be more strained in this sense. However from the through space C-C distances it is observed that the exo form has less steric strains (longer through space distances). In this case, the endo form is the predominate product as it has a lower transition state energy, which means the secondary orbital overlap effect dominates in this case.
Further analysis on the endo and exo forms using B3LYP/6-31G*
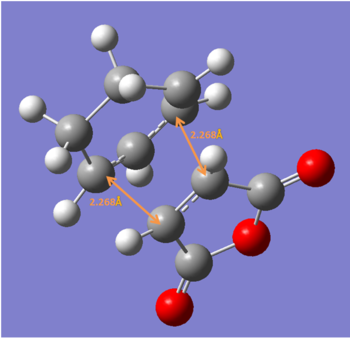
Endo
test molecule |
Exo
test molecule |
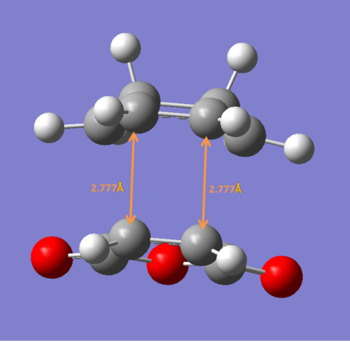
After reoptimization using B3LYP/6-31G*, the newly formed C-C bond is more consistent with synchronous bond formation.
Further Discussion
Diels−Alder cycloaddition of 4-Chloro-2(H)-pyran-2-one with methyl acrylate
[3]
i)Four substituted bicyclic lactone cycloadducts (5-endo, 6-endo, 5-exo, 5-endo) are analysed using QST2 method with AM1 semi-empirical molecular orbital method

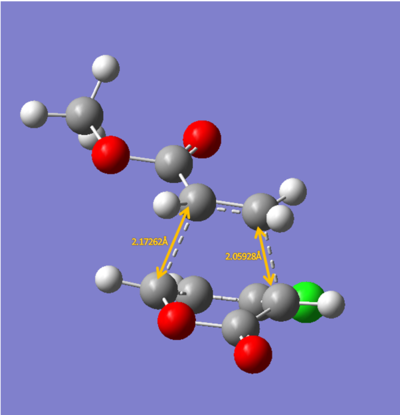
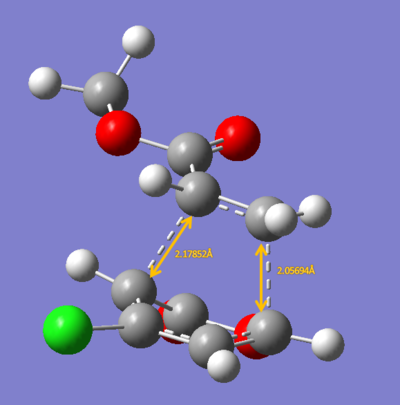
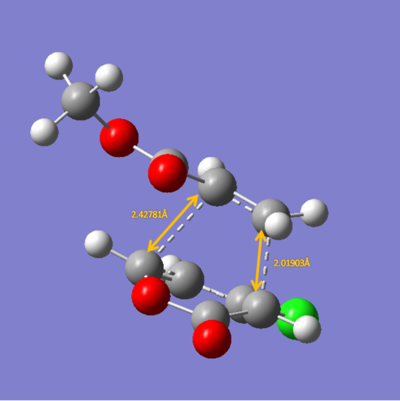
The energies calculated were shown in the table below
| Endo 5 | Endo 6 | Exo 5 | Exo 6 | |
| Energy/ a.u. | -0.1440 | -0.1476 | -0.1410 | -0.1413 |
The exo-5 form has the highest energy and the endo-6 form has the lowest energy.
The bond lengths calculated are quite different from the ones reported in the paper ,therefore further calculation were carried out using DFT method (B3LYP/6-31G*).
ii) The four transition states were reoptimized using DFT method (B3LYP/6-31G*).
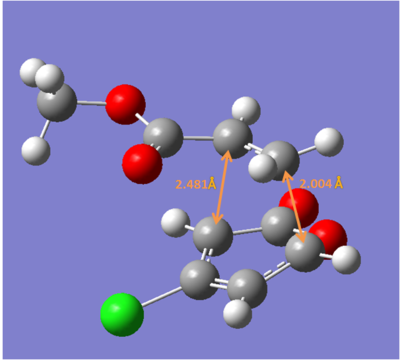
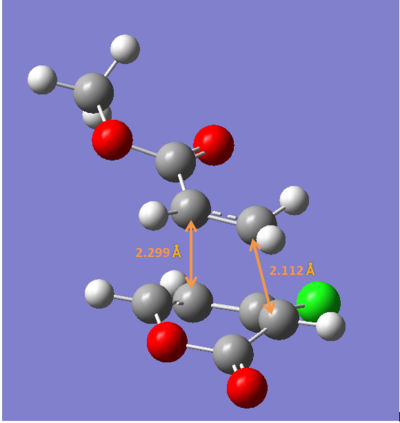
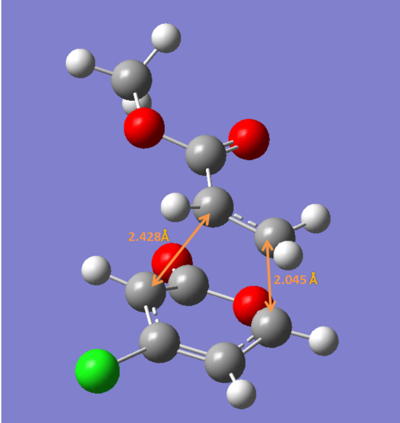
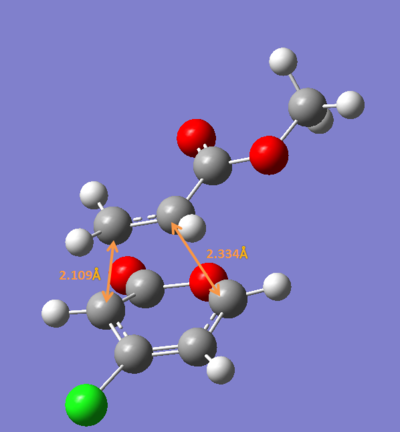
| Endo 5 | Endo 6 | Exo 5 | Exo 6 | |
| Energy/ a.u. | -1109.3930 | -1109.3941 | -1109.3902 | -1109.3910 |
The exo-5 form has the highest energy and the endo-6 form has the lowest enenrgy.
Concluding from the energies above, the endo-6 form would have the highest yield while the exo-6 form would have the lowest yiled. This consists with the experimental results.
References
- ↑ J.M.Baranowski "Bonds in carbon compounds" J.Phys.C:Solid State Phys.19(1986)4613-6421.
- ↑ Rowland RS, Taylor R (1996). "Intermolecular nonbonded contact distances in organic crystal structures: comparison with distances expected from van der Waals radii". J. Phys. Chem. 100 (18): 7384–7391.
- ↑ Kamyar Afarinkia, Michael J. Bearpark, and Alexis Ndibwami,Computational and Experimental Investigation of the Diels-Alder Cycloadditions of 4-Chloro-2(H)-pyran-2-one J. Org. Chem., 2003, 68 (19), 7158–7166