Rep:Mod:yg5515
NH3
summary
| molecule name | NH3 |
| calculation method | RB3LYP |
| Calculation type | FREQ |
| Basis set | 6-31G(d,p) |
| E(RB3LYP) | -56.55776863 a.u. |
| RMS Gradient Norm | 0.00000478 a.u. |
| point group | C3V |
| bond length | 1.01797 |
| bond angle | 105.743 |
item table
| item | value | threshold | converged? |
|---|---|---|---|
| maximum force | 0.000004 | 0.000450 | Yes |
| RMS force | 0.000004 | 0.000300 | Yes |
| maximum displacement | 0.000071 | 0.001800 | Yes |
| RMS displacement | 0.000034 | 0.001200 | Yes |
Predicted change in Energy=-5.828496D-10 Optimization completed.
NH3 |
The optimisation file is liked to here
frequency analysis
Questions:
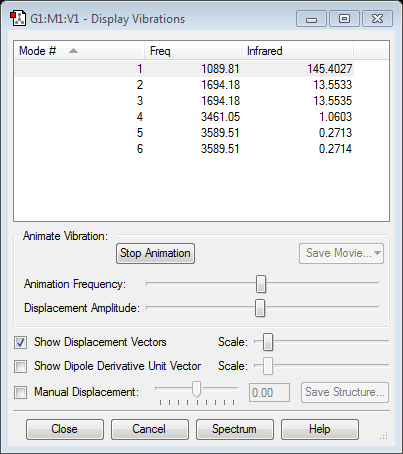
- 6 modes are expected from the rule, however, from the experimental observation, two pairs of the vibrations are degenerate.
- modes 2 and 3, 5 and 6 are degenerate
- bending has lower energy than stretching. Modes1, 2,and 3 are bending which correspond to lower frequency. Modes 4,5, and 6 are stretching which have higher frequency. It can be confirmed by running the animation.
- Modes 1 and 4 are highly symmetric
- mode 1 is umbrella mode.
- 4 bands are observed. The two different modes which have frequencies 3461 and 3589 are low in intensity.
charge analysis
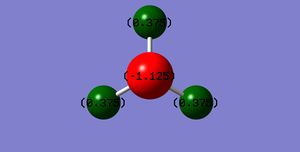
N:-1.125
H:0.375
The result is expected, because N is more electronegative than H, it draws electrons towards itself. So the energy of N should be more negative than H.
N2
summary
Name: N2
calculation type:FREQ
calculation method :RB3LYP
Basis set:6-31G(d.p)
E(RB3LYP):-109.52412868 a.u.
RMS gradient:0.00000060
point group : Dinfh
bond length :1.10550
bond angle :180
item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.400898D-13 Optimization completed.
N2 |
The optimisation file is liked to here
frequency analysis
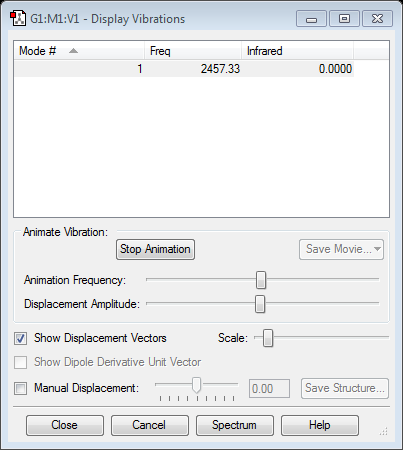
questions:
N2 shows one mode only.
H2
summary
Name: H2
calculation type:FREQ
calculation method :RB3LYP
Basis set:6-31G(d.p)
E(RB3LYP)-1.17853936 a.u.
RMS gradient:0.00000017
point group : Dinfh
bond length :0.60000
bond angle :180
item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13 Optimization completed.
H2 |
The optimisation file is liked to here
frequency analysis
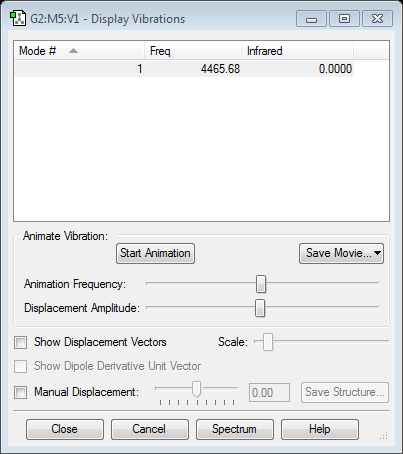
question:
only one mode is observed.
Energy calculation
• E(NH3)= -56.55776863
• 2*E(NH3)= -113.11553726
• E(N2)= -109.52412868
• E(H2)= -1.17853936
• 3*E(H2)= -3.53561808
• ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557907a.u. / -146.48KJ/mol
The energy change is negative. This is an exothermic reaction and it is energetically favorable.
My project molecule: H2O
summary
H2O optimization
File Name yg5515 H2O
File Type .log
Calculation Type FREQ
Calculation Method RB3LYP
Basis Set 6-31G(d,p)
Charge 0
Spin Singlet
E(RB3LYP) -76.41892999 a.u.
RMS Gradient Norm 0.00684281 a.u.
Imaginary Freq
Dipole Moment 1.9531 Debye
Point Group C2V
item table
Item Value Threshold Converged?
Maximum Force 0.000099 0.000450 YES RMS Force 0.000081 0.000300 YES Maximum Displacement 0.000115 0.001800 YES RMS Displacement 0.000120 0.001200 YES Predicted change in Energy=-1.939669D-08 Optimization completed.
H2O |
The optimisation file is liked to here
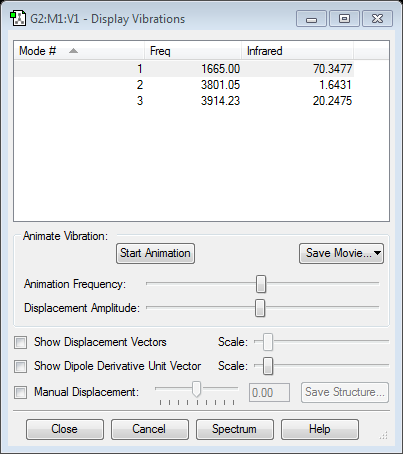
frequency analysis
Totally, there are three modes. The first mode is bending. The socond and the third are symmetric and unsymmetrical stretchings.
charge distribution
O is more electronegative, it draws the electrons towards itself, so the charge of O is negative.
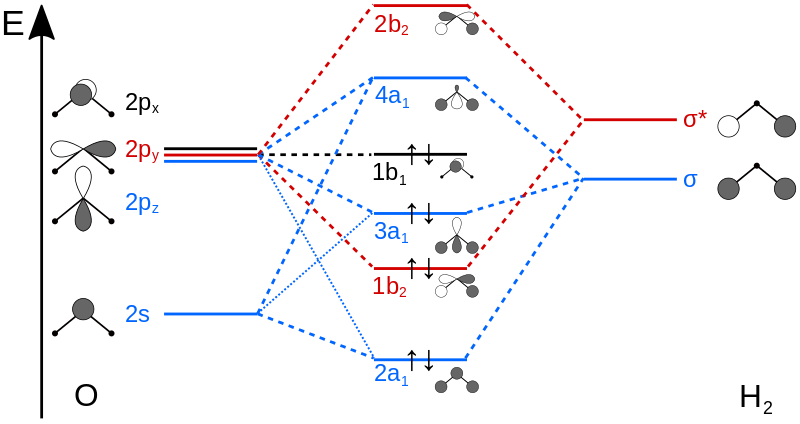
MO analysis
MO diagram
The electronic configuration of O is 1S2 2S2 2P4 H: 1S2
http://www1.lsbu.ac.uk/water/h2o_orbitals.html https://en.wikipedia.org/wiki/Molecular_orbital_diagram
MO images
This is the orbital which has the lowest energy. The two electrons are all from the 1S orbital of the O. Orbitals which have the same symmetry and are closer in energy are allowed to mix to generate new sets of molecular orbitals. Because the 1S electrons are too low in energy, they are not involved in the orbital mixing. The orbital is the 1a1 in the Fig.1.
This occupied bonding orbital is 2a1 in the figures. Mixing of the oxygen 2s AO and the hydrogen σ MO generate the MO orbital.
1b2 is an occupied bonding MO and it is from mixing of the oxygen 2py AO and the hydrogen σ* MO.
Mixing of the oxygen 2pz AO and the hydrogen σ MO generate the occupied bonding 3a1 MO.
This is 1b1 nonbonding MO from the oxygen 2px AO (the p-orbital perpendicular to the molecular plane). Importantly, this is the HOMO.
It should be noted that MO mixing can occur. If two molecular orbitals have the correct symmetry and similar energy they can mix to produce two new molecular orbitals. 2a1 and 3a1 can mix. 2a1 and 4a1 can also mix.