Rep:Mod:ydf2715ts
transition states and reactivity
Introduction
A potential energy suface is often used as a tool to support the analysis of chemical reaction dynamics and molelcular geometry. The stationay points, the points with a zero gradient, are mostly meaningful. The reactants and products correspond to the minima while the saddle points represent the transition states, which is the highest energy point on the reaction coordinate (the minimum energy path between the reactants and the products). When evaluating the necessary points on a potential energy surface, the first and second derivatives of the energy with respect to poision are usually used, which correspond to the gradient and the curvature respectively. The total gradient of a potential energy surface will be zero. For linear molecules, there are 3N - 5 vibrition modes while for non-linear molecules, there are 3N - 6 vibration modes. On the other word, there are 3N - 6 degrees of freedom and each one corresponds to a normal mode, which can be visualised using a classical harmonic oscillator model. [1]

Located in the minima of the potential energy surface, the reactants and products are stable and tend to stay where they are and vibrate constantly unless sufficient energy is given to help overcome the activation barrier. The transition state, on the other hand, is semi-stable and is ready to vibrate downhill towards the energy minima, i.e. the reactants and the products. The vibration modes of transition states give a negative force constant and the modes of reactants and products give a positive force constant, which makes it easy to distinguish the formars and the latters using the curvature, the second deravative of the energy with respect to bond distances.(∂2V(ri)/∂ri2).
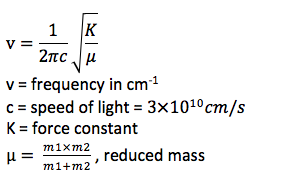
A positive value indicates a minimum, i.e. the reactants or the products, while a negative value indicates a maximum, a transition state. The frequency calculation is also used as a tool to confirm the position of a structure on the potential energy surface. Since the square root of the force constant is used to calculate the frequency, the second derivative of gradient can also distinguish reactants/products and transition states. The chemical pathways can be divided into two kinds, the thermodynamically controlled and the kinetically controlled ones. Under thermodynamic conditions, more stable products are afforded as the dominate product and the reaction is reversible, which makes the energy level of the transition states not important. On the other hand, under kinetic conditions, the reaction is irreversible and the energy of transition states is determinant. The lower the activation energy, the faster the kinetic product can form. Therefore, a transition state of lower energy is required to make the most rapidly afforded products predominate in kinetically controlled reactions. [3]
Nf710 (talk) 11:19, 11 January 2018 (UTC) This is all correct in 2D, but as you have said the Gaussian PES is in 3N-6 degrees of freedom. As a Minimum has a positive second derivative in all dimensions. Whereas A TS as Positive in all except one which is negative and that is the reaction coordinate. This data can be found from the eiegen values of the hessian matrix
Nf710 (talk) 00:26, 12 January 2018 (UTC) OK so you have clearly understood second derivatives. but you have not made the connection between this and the degrees of freedom which is the basis which we chose to represent the PES. A minimum has all second derivatives in all degrees of freedom (the 3N-6 dimensions) as positive. Whereas a TS has positive for all except one which is negative and this is the reaction coord. This info comes from the eigen values of the Hessian Matrix. You need to make sure you talk about how many dimensions the PES is in.
Exercise 1: Reaction of butadiene with ethylene
Below presents the reaction scheme for the cycloaddition reaction between 1,3-butadiene and ethylene.
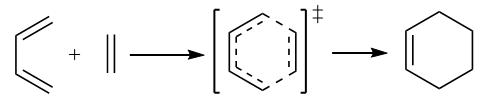
The reactants are optimised at the PM6 level. In this diels alder reaction, the cis geometry of butadiene gives rise to the correct orbital overlap to have the reaction take place. As the structure of the product is wholy predictable, method 3 in the tutorial was used to construct the structure of the transition state in this reaction after the optimizition of the product at PM6 level. The distance of 2.2Å was an intermidiate value between a normal single C-C bond (1.54Å) and the Van der Waals distance between two carbon atoms (3.4Å) and was used in C-C bond formation for the separation between the terminal carbons in both alkenes. After confirming the guess TS structure was correct, the guess structure was reoptimised with a more accurate method, B3LYP/6-31G(d), and then used to run a IRC.
Optimized structures
Below presents the optimized structures of the reactants, product and the transition state.
| Table 1. Optimized structures by PM6 calculation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,3-butadiene | Ethylene | Transition State | Cyclohexene | ||||||||
confirmation of TS
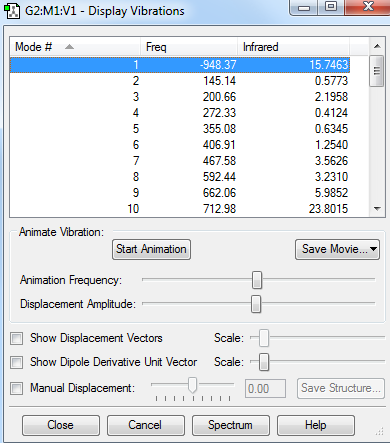
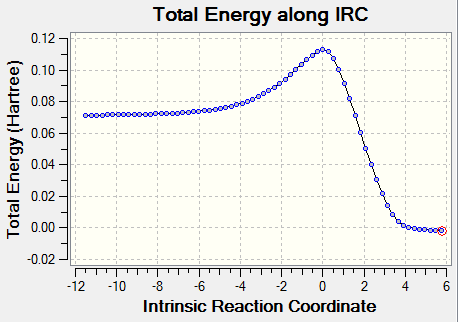
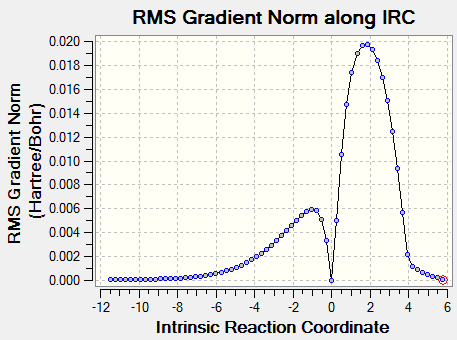
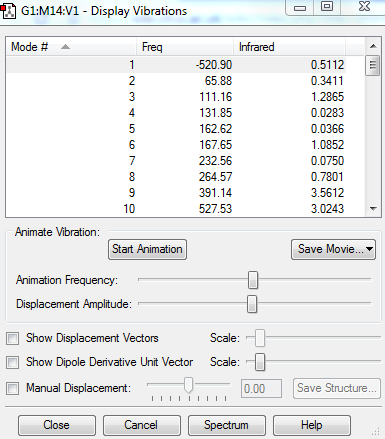
The vibrition modes of the TS and the IRC spectra are shown in the | vibration analysis part. There is only one negative value of the vibration, which represents the saddle point, i.e. the transition state, and the animation corresponds to the right reaction coordination, which indicates that the guess TS has the right structure. The IRC gave more direct proof of a correct TS structure by showing the correct reaction coordinate with minimum energy pathway. As shown in Figure 5, the energy of reactants go up to reach the level of transition state, crossing the activation barrier to for the products. The RMS gradient Norm along IRC shows that the gradients of reactants, products and the TS are all equal to zero.
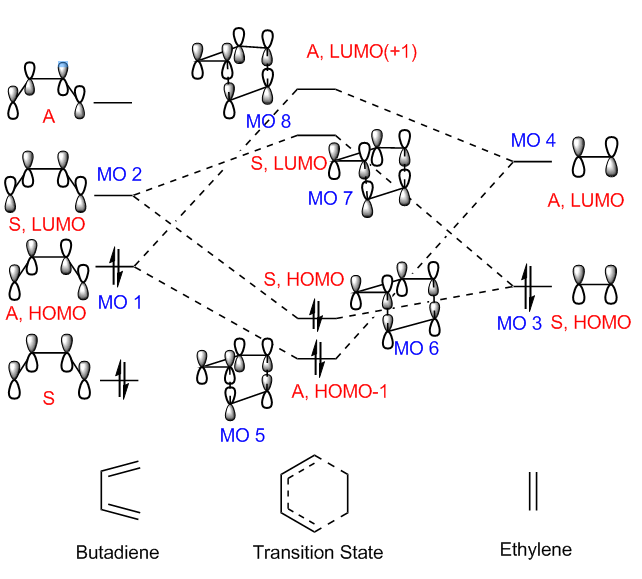
MO diagram and HOMO-LUMO orbitals
Below presents the MO diagram and HOMO and LUMO of reactants and transition state.
(Fv611 (talk) Your MO combinations are correct, but you are not representing the true relative energies of the TS MOs. As this is the MO diagram for a TS structure, the TS HOMO and HOMO-1 are actually higher in energy than the reactant HOMOs, while the TS LUMO and LUMO+1 are lower in energy than the reactant LUMOs.)
The capital letters A and S correspond to 'antisymmetric' and 'symmetric' respectively. The combination of the MOs from the reactants and the formation of the MOs from the TS are clearly presented above. The HOMO-LUMO gap of butadiene is smaller than that of ethylene as a result of its electron sufficient property. In the stage of TS, the electrons are rearranged to spins and geometries that can better delocalise the whole molecule.
As stated in Woodward Hoffmann rule, the total number of (4q+2)s + (4r)amust be odd in order to allow bimolecular cycloaddition reaction to take place. 'q' and 'r' represent integers starting from zero. The suffix 's' represents supprafacial (new bonds on the same face) and 'a' represents antarafacial (opposite faces are involved).[4] Below presents the [π4s + π2s] cycloaddtion in this investigated reaction.
(4q+2)s + (4r)a
=1 + 0
=1 => odd result, thermally allowed when q = r = 0
From the table above, it can be clearly concluded that only the MOs with the same symmetry are allowed to combine with each other to give a TS MO with the same symmetry. The symmetric HOMO of ethylene (MO3) interacts with symmetric LUMO of butadiene (MO 1) to afford symmetric HOMO (MO 6) and LUMO (MO 7) of TS. The antisymmetric LUMO of ethylene (MO 4) interacts with antisymmetric HOMO of butadiene (MO 1) to afford antisymmetric HOMO-1 (MO 5) and LUMO+1 (MO8) of TS. It is easy to understand as the symmetries have be matching since the combination of antisymmetric and symmetric orbitals gives rise to a zero overlap integral.
symmetric - symmetric interaction ==> non zero orbital overlap integral antisymmetric - antisymmetric interaction ==> non zero orbital overlap integral symmetric - antisymmetric interaction ==> zero orbital overlap integral
Therefore, with only the MOs with the same symmetry combining together, the reaction between 1,3-butadiene and ethylene is thermally allowed.
Bond length Analysis
The C-C bond lengths of the reactants, product and the TS are presented in the table above. The typical C-C bond lengths and the Van der Waals radius of the C atom are presented below.
| Table 4. typical C-C bond length [5] | |||||
|---|---|---|---|---|---|
| C-C bond type | sp3 - sp3 | sp3 - sp2 | sp2 - sp2 | vdW radius of C | vdW distance of 2 C |
| Bond length | 1.54Å | 1.50Å | 1.47Å | 1.70Å | 3.40Å |
In the TS, there are three types of bonds, the partly breaking double bond, the partly forming single bond and the partly forming double bond. As the reaction progresses, the bond lengthes of the double bonds in butadiene (C1 - C2 and C3 - C4) increase from 1.34Å to 1.38Å in the TS and then to 1.50Å in the product. This is due to the change in the carbon hybridisation from sp2 to sp3 and the formation of the new σ bonds. The bond lengths of the two partly formed single bonds (C3 - C4 and C1 - C6) in the TS is 2.11Å, which is between the value of typical sp3 C-C bond length and the Van der Waals distance of 2 C atoms. The double bond in ethylene (C5 - C6) is weakened from 1.34Å to 1.38Å in the TS and then to 1.54Å in the product. The newly formed double bond (C2 - C3) grows from 1.47Å in butadiene to 1.41Å in the TS and then to 1.34Å in the product. The decrease of bond length reveals the additional π overlap between the two carbons, which largely strengthens the bond.
Vibration analysis
Below presents the vibration that corresponds to the reaction path at the transition state.
There is only one negative value of the vibration, which represents the saddle point, i.e. the transition state and the vibration frequency to reaction path is -948.37cm-1. From the animation, it is clear that the formation of the two C-C bonds is synchronous, since the two carbons in each pair move towards each other at the same rate.
Exercise 2: Cycloaddtion of cyclohexadiene and 1,3-dioxole
The Diels - Alder reaction is an organic [4+2] cycloaddition reaction between a conjugated diene and a substituted alkene, usually termed the dienophile, to produce a substituted cyclohexene system. In Exercise 2, the pericyclic reaction between cyclohexadiene and 1,3-dioxole was investigated. Below presents the reaction scheme.
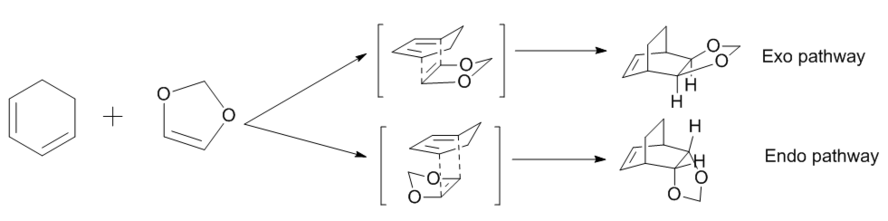
Resulting from the significant stereoselectivity and regioselectivity of the 6-membered ring genenrated, this reaction is of great synthetic utility and has been a research topic fasinating for many years. The frontier molecular orbital model of cycloaddition reactions was raised by Kenichi Fukui[6], and is an approximation that remarkably succeeds in rationalizing the chemical properties in Diels - Alder reactions. It is assumed that it is the interactions of the HOMO of one molecule with the LUMO of the second molecule that determines the chemistry of the conjugated π systems in the reaction.[7] In the reaction between cyclohexadiene and 1,3-dioxole, the ring structures in both reactants give rise to two products, the exo and the endo products. The stereoselectivity was also studied in this exercise.
Optimized structures, vibration results
As the structures of the products are predictable, method 3 in the tutorial was used to construct the structure of the TSs in this reaction after the optimizition of the products. As shown After confirming the guess TS structure was correct, both endo and exo TSs were optimised at PM6 level first and then reoptimised with B3LYP/6-31G(d). Frequency calculation and the IRC were also carried out in the experiment. Below presents the structures for reactants, TSs and products after optimization.
There is only one negative value in each vibration, which corresponds to the saddle point, i.e. the transition state, and indicates that the guess TS has the right structure.
MO diagrams and HOMO-LUMO orbitals
(Fv611 (talk) Very nice MO diagrams, and very nice discussion on the difference between the endo and exo case in terms of electron demand in the next section. Well done!)
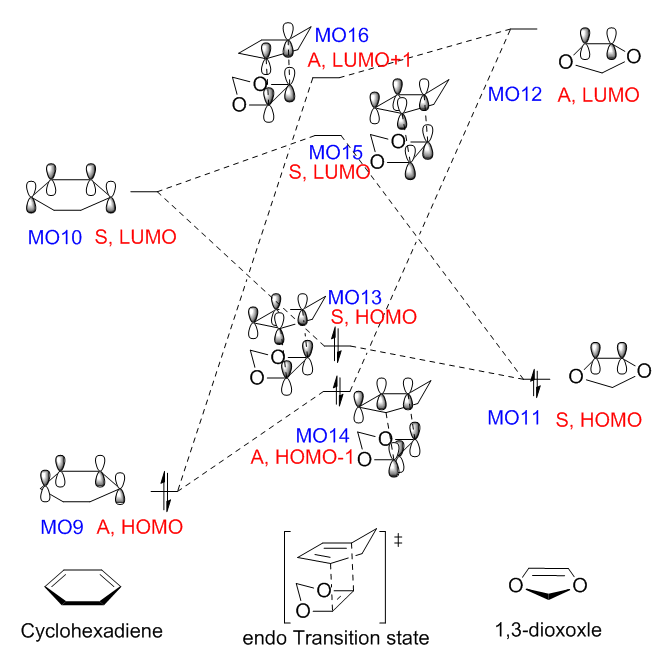
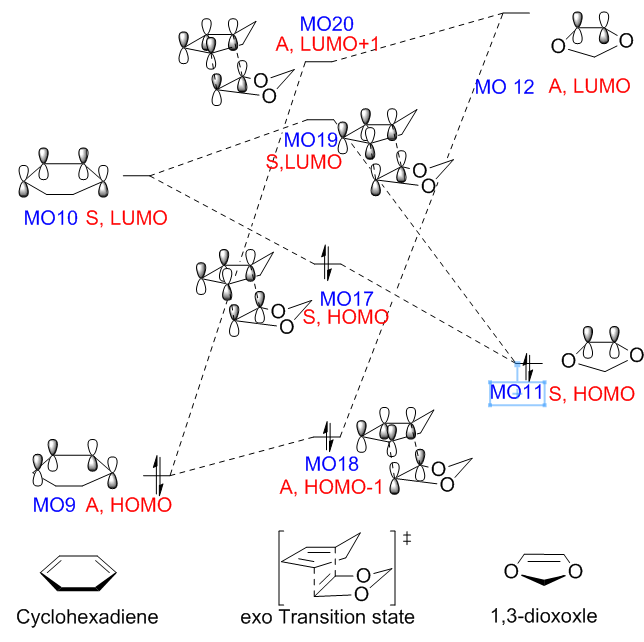
The capital letters A and S correspond to 'antisymmetric' and 'symmetric' respectively. The combination of the MOs from the reactants and the formation of the MOs from the TS are clearly presented below. The occupied and unoccupied orbitals associated with the DA reaction for both TSs were located by symmetry. The HOMO/LUMO orbitals of the chemicals involved in this reaction and the two MO diagrams for both endo and exo pathways are all presented in the table below.
| Chemicals and pathways | HOMO (HOMO - 1) | LUMO (LUMO + 1) | MO interactions |
|---|---|---|---|
| Cyclohexadiene |  |
 |
  |
| 1,3-dioxole | 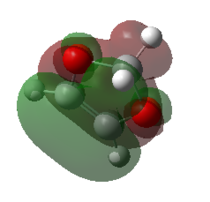 |
 | |
| Endo TS | 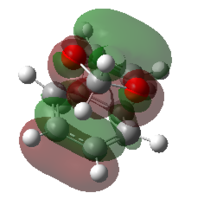 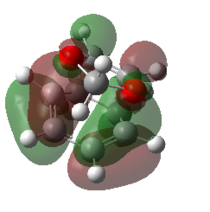 |
  | |
| Exo TS | 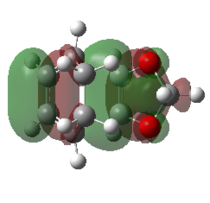 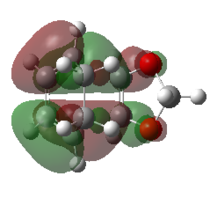 |
  |
Normal or inverse demand DA reaction ?
As mentioned in the first paragraph, Diels - Alder reaction is a concerted [4+2] cycloaddition reaction between a conjugated diene and a substituted dienophile. The DA reaction can be divided into two types depanding on the relative energy level of the frontier MOs during the formation of the new single bonds. In normal DA reactions, TSs have highly stabilizing interactions between the HOMO of diene and the LUMO of dipolarophile, which results from the small energy gap between the HOMO-LUMO. Resulting from the absence of a largely stabilizing interaction, the electron - sufficient alkenes are unreactive with simple dienes. On the other hand, Diels - Alder reactions with 'inverse electron demand' arise under the circumstance that the interaction between LUMO of diene and HOMO of dienophile is greater, i.e. the energy gap is small than that between HOMO of diene and LUMO of dieophile. Since electron sufficiency can higher the HOMO of dienophile and electron defficiency can lower the LUMO of diene, electron rich dienophiles and electron defficient dienes would have smaller HOMO-LUMO gap and accelerate the reaction rate of an inverse electron demand DA reaction.[8]
For the DA reaction between cyclohexadiene and 1,3-dioxole, the energy gap between the LUMO of dienophile (MO12, 0.041a.u.) and the HOMO of diene (MO0, -0.326a.u.) is 0.367au. The LUMO of diene (MO10, 0.021a.u.) and the HOMO of dienophile (MO11, -0.192a.u.) have a smaller energy gap (0.213a.u.), which enhance the inverse electron demand DA reaction to form MO 13 and MO 15 (as presented in table 7). This is easy to understand, as the 1,3-dioxole benefits from the the electro - releasing oxygen atoms and is highly electron rich.
Nf710 (talk) 11:39, 11 January 2018 (UTC) This is a good understanding. You could have shown this quantitatively by doing a an energy calculation with both reactants on the same potential energy surface.
Energy analysis
| Compounds | Energy calculated at PM6 level / Hatress | Energy calculated at PM6 level / kJmol-1 | Energy calculated using B3LYP/6-31G(d) /Hatree | Energy calculated using B3LYP/6-31G(d) x 105/ kJmol-1 |
|---|---|---|---|---|
| Cyclohexadiene | 0.1171 | 307.39 | -233.430962 | -6.12756276 |
| 1,3-dioxole | -0.0513 | -134.66 | -267.116106 | -7.01179778 |
| sum of reactants | 0.0658 | 172.73 | -500.547068 | -13.1394904 |
| Endo TS | 0.1380 | 362.25 | -500.427867 | -13.1362315 |
| Exo TS | 0.1391 | 365.14 | -500.506565 | -13.1382973 |
| Endo product | 0.0377 | 98.96 | -500.602493 | -13.1410815 |
| Exo product | 0.0379 | 99.49 | -500.601679 | -13.1407941 |
The kinetically favoured product is determined by the activation energy. The smaller the activation energy, the faster the product can be formed. For the thermodynamically favoured product, the reaction energy is a factor more important. Under thermodynamic conditions, the reaction is reversible and is an equilibrium. The more exothermic or less endothermic pathway will give more smaller (more negative) reaction energy and more stable product.
| Pathways with methods | Activation barrier (Ea) / kJmol-1 | Reaction energy / kJmol-1 |
|---|---|---|
| Endo (PM 6) | +189.52 | -73.77 |
| Endo(B3LYP) | +158.76 | -67.41 |
| Exo (PM 6) | +192.41 | -73.24 |
| Exo(B3LYP) | +167.29 | -63.84 |
Activation energy : +189.52 < +192.41 (PM6)
+158.76 < +167.29 (B3LYP) ==> Endo product more kinetically favoured.
Reaction energy : -73.77 < -73.24 (PM6)
-67.41 < -63.84 (B3LYP) ==> Endo product more thermodynamically favoured.
Consequently, the endo product is both kinetically and thermodynamically favoured in this reaction. This is easy to understand, as the exo product is kinetically more hindered and thermodynamically less stable. This conclusion can be further confirmed by considering the secondary orbital interactions. Below presents the HOMOs of both endo and exo TSs. The isovalue was set to 0.01 in Gaussiview.
| Endo TS HOMO (MO 13) | Exo TS HOMO (MO 17) | Discussion |
|---|---|---|
 |
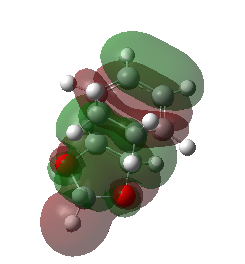 |
The secondary orbital interaction is the determinant factor of the dominate product in this reaction. As proved above, the endo pathway is kinetically more favoured, which means the corresponding activation energy is lower and the energy of endo TS is also lower than that of the exo TS. In the endo TS, the non - bonding p orbital on the oxygen atoms of 1,3 - dioxole interacts with the LUMO of cyclohexadiene, nOp → LUMO of cyclohexadiene, which strongly stablise the endo TS and lower the activation energy.
This seconday orbital interaction works on both the transition state and the final products. The endo product benefits from this interaction and is stabilised with a lower energy. Therefore the overall reaction energy of the endo pathway is more exothermic.
|
Nf710 (talk) 11:42, 11 January 2018 (UTC) There is evidence of steric clashing in the exo. Your energies are correct and you have come to the correct conclusion. You could have used some diagrams to explain your points better.
Exercise 3: Diels-Alder vs Cheletropic
Below presents the reaction scheme for the cycloaddition reaction between o-xylylene and SO2.
Similar to exercise 2, there are two pathways for this Diels - Alder reaction, the endo pathway and the exo pathway. The two reactants, the o-yxlylene and SO2 are sptimised at the PM6 level. During the experimental, the endo DA TS and the corresponding adduct were drawn. Method 3 was used to construct a guess exo TS structure. The newly formed bonds in the reaction were broken and the C-O separation was set at 2.0Å and the C-S separation was set at 2.4Å. After having the relative atoms frozen in space, optimization was carried out at the PM6 level to afford the guess exo TS structure. The guess TS structure reoptimised before the IRC and frequency calculations were carried out. After confirming the guess TS structure was correct, the reaction coordination with an IRC calculation for each reaction pathway were visalised. The reaction profile that contains relative heights of the energy levels of the reactants, TSs and products from the three reaction pathways were drawn in the analysis part.
(Don't call this the "experimental" Tam10 (talk) 12:35, 9 January 2018 (UTC))
Optimized structures
The TSs for the endo- and exo- Diels - Alder reaction and the Cheletropic reactions were all optimized at the PM6 level. Below presents all the optimized structures for reactants, TSs and products.
| Optimized reactants | Species | Diels-Alder Reaction | Cheletropic reaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endo pathway | Exo pathway | ||||||||||||
|
Transition State | ||||||||||||
|
Products | ||||||||||||
(Your choices for frames are seemingly random. Just be careful to select the frame numbers that correspond to the information that you want to display Tam10 (talk) 12:35, 9 January 2018 (UTC))
As shown in reaction scheme, one of the major driving force of the reaction between xylylene and SO2 is the recovery of the bezene ring and the aromatic chararter. Xylylene is often produced through the pyrolysis reaction of xylene and the significant thermal energy destroys the bezene structure. According to Huckel's rule, a molecule has to have the requirements below satisfied, to be considered as aromatic.[9]
1. the molecule must have 4n + 2 electrons in p orbitals 2. the molecule must be planar 3. the molecule must be cyclic 4. the molecule must have a continuous ring of p orbitals
In spite of the planar structure with conjugated double bonds, xylylene is considered as a non - aromatic molecule. Although it has 6 electrons in total, only 4 of them are in the ring, and with the presence of a diradical, the molecule is remarkably unstable. The non - aromatic character gives rise to a relatively high energy π system, which is readily reactive.
(Xylyene has 8 π electrons: one for each sp2 hybridised carbon Tam10 (talk) 12:35, 9 January 2018 (UTC))
IRC analysis
Below presents the IRC calculation and the reaction trajectories for the three reactions. The energy changes along the reaction coordinate and the way reactants approaching each other to form the products are all clearly shown below.
| Reaction pathways | IRC calculation | IRC animation |
|---|---|---|
| DA - Endo | 
|
 |
| DA - Exo | 
|
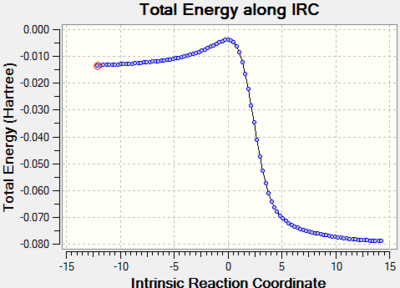 |
| Cheletropic | 
|
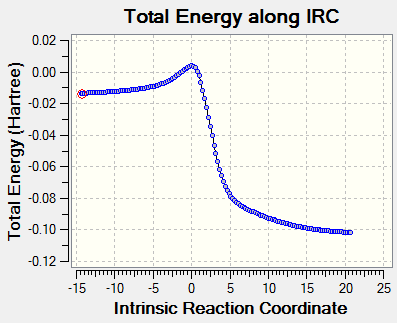 |
As mentioned above, xylylene is highly unstable. The IRC calculation provides proofs for this. From graphs of total energy against IRC, the large reaction energy indicates that the three reactions are all highly exotermic and the small activation energy indicates the high reactivity of xylylene. The three products benefit from the aromaticity (as there are 6 π electrons in the cyclic π system in the ring, 4n+2, n=1) and are all stablized by the the strong resonance in the benzene ring. During the course of the reaction, the changes of the bond lengths in the 6 - membered ring are presented below.
| Table 13. Changes of bond lengths in the reaction | ||||
|---|---|---|---|---|
| C-C bond type | Xylylene | Exo/Endo C-C bond | Cheletropic C-C bond | |
| C=C bond | C-C bond | |||
| Bond length | 1.35Å | 1.46Å, 1.47Å, 1.49Å | 1.47Å | 1.47Å |
As mentioned in Exercise 1, the C-C bond of sp2 - sp2 hybridisation is 1.47Å, which lies between C-C single bond (1.54Å) and C=C double bond (1.34Å). From table 12, it is clear that the bond lengths of the C-C bonds in the products are all 1.47Å. The strong delocalisation of the π electrons in the benzene ring strengthens the C-C bonds in the products.
Energy analysis
Below presents the activation energies and reaction energies for the three pathways.
| Measurement | Energies / kJmol-1 | ||
|---|---|---|---|
| DA | Cheletropic reaction | ||
| Exo | Endo | ||
| xylylene | + 467.89 | ||
| Sulfur dioxide | - 312.01 | ||
| Sum of reactant energy | + 155.88 | ||
| Transition states | + 241.76 | + 238.02 | + 259.98 |
| Product | + 56.37 | + 57.02 | + 0.0105 |
| Activation barrier (Ea) /kJmol-1 | + 85.88 | + 82.14 | + 104.10 |
| Reaction energy /kJmol-1 | - 99.51 | - 98.86 | - 155.87 |
The kinetically favoured product is determined by the activation energy. The smaller the activation energy, the faster the product can be formed. For the thermodynamically favoured product, the reaction energy is a factor more important. Under thermodynamic conditions, the reaction is reversible and is an equilibrium. The more exothermic or less endothermic pathway will give more smaller (more negative) reaction energy and more stable product.
As the endo DA reaction has the lowest activation energy, the endo DA reaction is the kinetically favoured reaction. And exo DA the second, Cheletropic reaction the third thermodynamically favoured.
As the reaction energy of Cheletropic reaction is most exothermic, the Cheletropic reaction is the thermodynamically favoured reaction. And exo DA the second, endo DA reaction the third thermodynamically favoured.
(Why would the cheletropic reaction have the highest barrier and reaction energy? Tam10 (talk) 12:35, 9 January 2018 (UTC))
Below presents the reaction profile that contains relative heights of the energy levels of the reactants, TSs and products from the endo- and exo- Diels - Alder reactions and the cheletropic reaction.
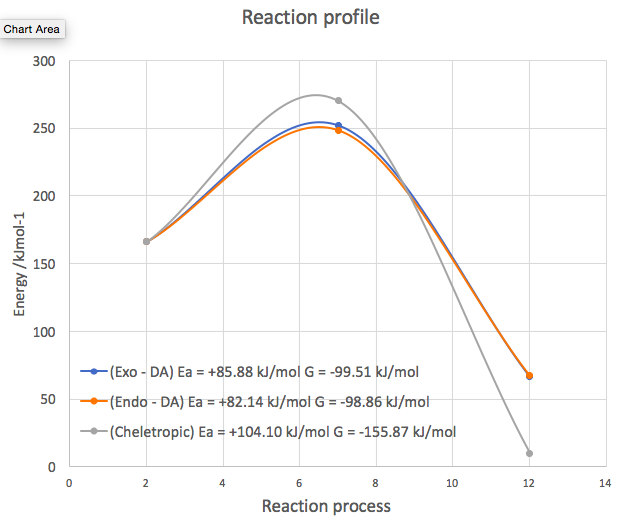
(Please don't use curves for these diagrams. 1) You are interpolating using 3 points which is very inaccurate 2) You've created points that are higher than the TS energy 3) the gradients are non-zero at all of your stationary points on this diagram. It's much better to just draw straight lines, as this indicates you are not providing gradient information with your diagram Tam10 (talk) 12:35, 9 January 2018 (UTC))
Conclusion
In this experiment, a variety of pericyclic reactions were located and their transition structures are characterized. Molecular orbital-based methods were used to numerically solving the Schrödinger equation and locate the transition structures based on the local shape of a potential energy surface. The reaction coordinates, reaction energies and activation barrier heights were also calculated. The roles of sterics and secondary orbital interactions in determining the kinetic and thermodynamic products of a reaction was also discussed.
For the concerted cycloaddtion reaction between 1,3-butadiene and ethylene, the bond formation is synchronous as the two carbons in each pair move towards each other at the same rate. It was also confirmed that only the MOs with the same symmetry are allowed to combine with each other to give a TS MO with the same symmetry. In exercise 2, the reaction between cyclohexadiene and 1,3-dioxole has an inverse electron demand. From the energy analysis, the endo pathway was proved to be more kinetically and thermodynamically favoured as a result of secondary orbital interactions. In exercise 3, there are three different reaction pathways for xylylene and sulfur dioxide, the endo/exo Diels - Ader reaction and the Cheletropic reaction. From the energy analysis, the endo product was proved to be more kinetically favoured and the cheletropic product was more thermodynamically favoured. A reaction profile was used to help read the reaction more directly.
Reference
- ↑ E. G. Lewars, Computational Chemistry, 2010, 9–43
- ↑ D. L. Pavia, G. M. Lampman, G. S. Kriz and J. R. Vyvyan, Introduction to spectroscopy, Cengage Learning, Australia, 2015.
- ↑ P. J. Atkins, J. D. Paula and J. Keeler, Atkins Physical Chemistry, Oxford Univ. Press, Oxford, 2017.
- ↑ P. Geerlings, P. W. Ayers, A. Toro-Labbé, P. K. Chattaraj and F. D. Proft, Accounts of Chemical Research, 2012, 45, 683–695
- ↑ E. M. Popov, G. A. Kogan and V. N. Zheltova, Theoretical and Experimental Chemistry, 1972, 6, 11–19.
- ↑ K. Fukui, Frontier Orbitals and Reaction Paths World Scientific Series in 20th Century Chemistry, 1997, 150–170.
- ↑ K. N. Houk, Accounts of Chemical Research, 1975, 8, 361–369.
- ↑ K. Yang, Z. Yang, Q. Dang and X. Bai, European Journal of Organic Chemistry, 2015, 2015, 4344–4347.
- ↑ Organic Chemistry, Springer Verlag, 2014.
Links to the log files
Exercise 1
The IRC calculation for the transition state : File:Yf 1 TSIRC.LOG
Exercise 2
| Chemicals | Methods | |
|---|---|---|
| PM 6 | B3YLP | |
| Cyclohexadiene | File:YFCYCLO.LOG | File:YF631CYC.LOG |
| 1,3-dioxole | File:YFDIO1.LOG | File:YF631DIO.LOG |
| TS of EXO | File:YfEXOTS3.LOG | File:YF631TSEXO.LOG |
| TS of Endo | File:YfENDOTS3.LOG | File:YF631TSENDO.LOG |
| Exo product | File:YfEXO3.LOG | File:YF631EXO.LOG |
| Endo product | File:YfENDO3.LOG | File:YF631ENDO.LOG |
Exercise 3
| Diels Alder Reaction | Cheletropic Reaction | |
|---|---|---|
| Exo-pathway | Endo-pathway | |
| File:YfEXOIRC.LOG | File:YfENDOIRC.LOG | File:YfCHEIRC.LOG |