Rep:Mod:yas1c
Yasser Farhat 1C: Molecular modelling and assignment of configuration of an Epoxide
All optimizations will be carried on using MMFF94s method on Avegadro, unless otherwise stated. Calculated energies will be given to 2 d.p
Part1: Conformational analysis using Molecular Mechanics
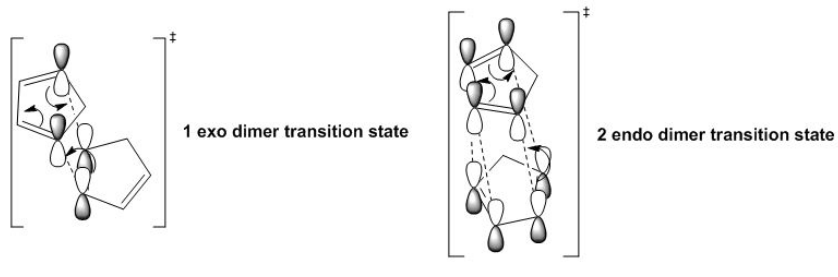
Cyclopentadienyl dimers
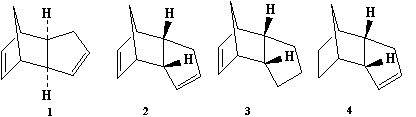
Cyclopenyldiene typically undergoes a dimerization via a Diels-Alder cycloaddition [1]. The products are an exo or endo type product, under standard conditions, research show the endo dimer predominates (molecule 2 shown to the right). In this section I was asses the reasoning behind the selectivity and control of the endo product. Below shows the cycloaddition process for each dimer:
The endo-isomer is the predominant product of the dimerisation process, as shown by Woodward and Hoffmann. the kinetic endo-isomer is predominant in this dimerisation reaction, suggesting that the reaction is kinetically controlled. This can be explained by invoking the relative stabilities of the transition states of the molecules. From literature [2], it is explained that favourable secondary orbital interactions are present in the endo-isomer, which is due to the interaction of frontier orbitals of the diene and dienophile components, lowering the energy of the transition state, thereby favouring the endo-isomer to be formed predominantly.
The exo-isomer is favoured due to a lower degree of steric congestion while the endo-isomer enjoys stabilisation due to orbital overlaps. The exo-isomer comprises only two pairs of orbital interactions, while the endo-isomer benefits from four pairs of such interactions, leading to a lower energy (stabilised) transition state, and under kinetic control which is affected directly by the relative energies of transition states, the endo-isomer is predominantly formed. The thermodynamically more stable product is the exo-isomer, and as seen from this example, the higher energy endo-isomer is formed, showing this reaction is under kinetic control in which orbital interactions predominates, leading to the endo-isomer which has a lower energy transition state.

Optimization results of each dimer is summarised below:
| Contribution | Exo(1) | Endo(2) |
|---|---|---|
| Bond stretching energy | 3.54 | 3.47 |
| Angle bending energy | 30.77 | 33.19 |
| Van der Waals energy | 12.80 | 12.36 |
| Electrostatic | 13.01367 | 14.18 |
| Total energy | 55.37 | 58.19 |
Results show the endo product to have a greater energy (58.1907 kcal/mol) compared to that of the exo product (55.3734 kcal/mol), these results are conformed by a computational study performed by Caramella et al.in 2002[3]. As such the endo is predicted to be less thermodynamically stable, hence dimerisation is kinetically controlled, this also is conformed by Caramella et al. work, which states that for the highest energy transition state, which they term as 7N to form the endo dimer is 21.0kcal/mol, which is 2.9kcal/mol lower than the highest energy transition state of the exo product.
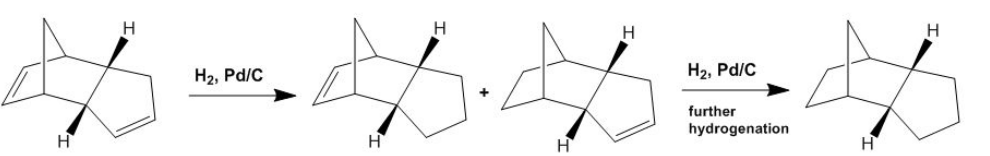
Hydrogenation of Endo Cyclopentadiene dimer
Cyclopentadiene is hydrogented in a stepwise process where one double bond is hydrogenated before the second double bond. Literature shows hydrogenated product 4 (from figure 1 above) is hydrogenated 1st. Below outlines the reaction scheme to hydrogenation process:
As performed above, the same molecular mechanics simulations where performed and the computed energy contributions for both product 3 and 4 are summarised below:
| Contribution | Product 3 | Product 4 |
|---|---|---|
| Bond stretching energy | 3.31 | 2.82 |
| Angle bending energy | 30.68 | 26.69 |
| Van der Waals energy | 13.28 | 10.64 |
| Electrostatic | 5.12 | 5.15 |
| Total energy | 50.72 | 41.26 |
As shown above product 4 is much more stable (by 9.46 kcal/mol) which explains the reason for the reason for its selective hydrogenation. The data above shows the two greatest contributions (Van da Waals and Angle Bending energy) are lowest in both cases for product 4 with respect to product 3. Its also observed product 3 has a positive torsional energy, this indicates product 3 has a greater strain and greater 1,3 dihedral clash.
Atropisomerism in a Taxol intermediate
Taxol is trademark mitrotic inhibitor of the drug paclitaxel used in cancer chemotherapy, first isolated by Monroe E. Wall and Mansuckh C. wani. It was first synthesised by Holton el al in 1988 via semisynthetic route from needles of T. baccata [4]. It has an isometric structure as shown in figure 2 shows three out the four rings in paclitaxel, the atropoisomerism was studied by Paquette [5].
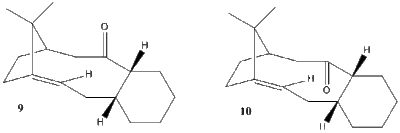
.The compound can be obtained in an oxy-cope rearrangement, which yields one of the isomers depending on other groups present in the reagent molecule. The isomers are isolable conformers due to restricted rotation around the single bond adjacent to the keto-group, given rise to the atropisomer nature. To investigate which astropisomer is the thermodynamic product, molecular mechanics is applied. table below summarises information:
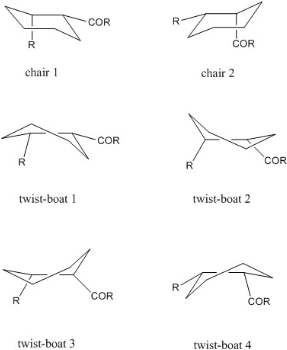
For the three conformers that states 'No structure found' was due to trail and error these conformers would alter to other found conformers. the chair 2 conformer. In the tables above is shows that for taxol 9 the lowest energy conformer is the chair 1 (from Figure 3) conformer, and for taxol 10 the lowest energy conformer is chair 2. For the lowest energy conformers atropisomer 9 and atropisomer 10, it is found that the beta-CH2 group to the carbonyl group is arranged in a anti-periplanar fashion. The most stable for all conformers is the atropisomer 10 chair 2 conformer, therefore it can be concluded that isomer 10 is more stable than isomer 9, hence a sample initially containing only 9 will after some time become a mixture of 9 and 10 with a (very large) excess of 10. The excess of isomer 10 will also determine the dominant product of a nucleophilic addition to the carbonyl group - during such reaction the nucleophile approaches the group with Burgi-Dunitz trajectory [6], so the dominant product will have the added nucleophile on the outside of the ring and the oxygen pointing down (as viewed in Figure 2). Functionalization of the double bond in the compound 9/10 proceeds slower than expected. Maier et al. [7] found in their MM1 computations that some "bridgehead" alkenes can have less strain than their "parent" (unsaturated) hydrocarbons. Here, energies of the saturated counterparts of 9 and 10 were calculated:
| conformation of the cyclohexane ring | saturated 9 | saturated 10 |
|---|---|---|
| Lowest chair conformation | 81.75 3D model | 71.51 3D model |
The energies of the saturated for the lowest conformers of atropisomers of 9 and 10 have a higher energies, this is due to greater 'strain' than there half-hygrodenated isomers. It is also noticed that the saturated chair 2 conformer of astropisomer 10 is lower in energy than saturated chair 1 astropisomer 9 conformer by the same amount of energy 10 kcal/mol. The second hydrogenation is higher in energy than the 1st indicated an endothermic process.
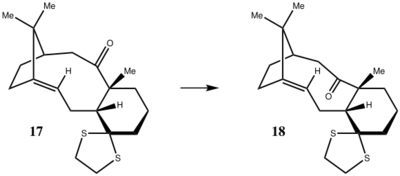
Its also been noted that both antropisomers react much slower than other alkene, there given the term 'hyperstable olefins' as they are a class of bridgehead alkenes[8] . The double bond in the bridgehead position causes alot of strain in cyclic molecules, it can adapt a very favourable conformation in larger polycycles. These compunds have been found to have negative olefin strain (OS) values, defined as the difference of the strain energy between the alkene and parent alkane, hence their stability. Inspecting the computed structures of compounds (17) and (18) below shows, that the double bond geometry is indeed close to that in an unstrained alkene. The reaction cannot be modelled using Molecular Mechanics as it involves bond breaking and making, however an ab-initio calculation at a modest level of theory could show the unfavourable energetics. [7]
Spectroscopic Simulation using Quantum Mechanics
To take the analysis of isomers 9 and 10 one step further, their derivatives, molecules 17 and 18, which are also isomers are also analysed. One of the isomers, molecule 18 was constructed and optimized on Avogadro exported to Guasssview to appended keywords and send to the HPC server. The proton and 13C NMR spectra of the compound was also calculated using computational chemistry methods and compared to that reported in literature. The structure of the molecule is shown in the figure below:
The geometry of the molecule was optimised to the density functional DFT level, job details are shown:
- Job type: Minimise
- Method: DFT/B3LYP
- Basis set: 6-31G, d,p polarisation
- Solvation model: CPCM/ chloroform
- First line of .gjf file after correction: # RB3LYP/6-31G(d,p) Opt SCRF=(CPCM,Solvent=chloroform)
- job was submitted to HPC server
The .fchk file of the optimisation was then retrieved and set as another input file:
- First line of .gjf file after optimisation: # mpw1pw91/6-31G(d,p) NMR SCRF=(CPCM,Solvent=chloroform)
- Job was resubmitted to HPC server
The chemical shifts calculated using these input files were then tabulated and compared to literature data [5]
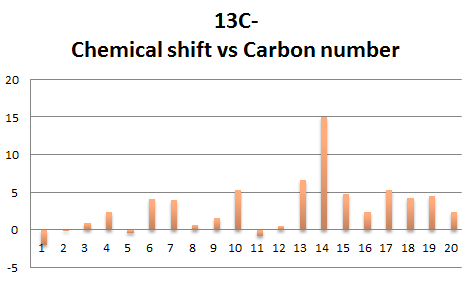
Below shows the compairson between the calculated and literature 1h NMR.
Comparison between calculated and literature chemical shifts where quite similar varying with approx 5 ppm of each other, an outlier from above can be seen is the carbon atom labelled 14 which as a difference of 15.12 ppm, this could be due to the carbon being connected to 2 larger S atoms, causing less accurated calculations to take place. During the optimisation of the molecule, in light of the work on molecule 10, the geometry of the cyclohexane six-membered ring was tilted for a better optimisation. The larger number of electrons that S has may be a contributing factor to this anomaly, and so can be the mismatch of the solvation system set for the optimisation and calculation. Another calculation was repeated using C6H6 (the one used in the literature source), but the results yielded were very similar and there was barely any improvement on the 15.12 ppm difference. As for the 1H NMR, the chemical shifts and number of signals calculated match more closely to that reported in literature, with the largest difference being 0.86 ppm for proton 26. Comparison has shown that the calculated values agree quite closely to that reported in literature, with the only significant anomalous piece of data being that of the 24th C in the 13C NMR. Possible reasons were suggested but it is unknown that which is in fact the main factor contributing to this difference.
Chiral Catalysis
The Chiral catalysts

Shi pre-catalyst: (molecule 21): The Shi-precatalyst contains only one anomeric centre and contains a cyclic ether. The equilibration with the other anomeric form is hindered due to the hydroxyl group being protected by the five-membered ring. The bond length of the ring oxygen and anomeric carbon is 1.408Å and the bond length to the other carbon is 1.414Å. The shortening of the anomeric C-O bond is insignificant, however the bond distance between the anomeric carbon and the oxygen atom involved in the 5-membered ring, which is 1.390Å is noticably shorter. Even shorter, 1.345Å and 1.380Å, are the bonds formed by the other oxygen atoms in that small ring. These contractions could be due to the geommetry of the five-membered ring.
Jacobsen pre-catalyst (molecule 23) : The phenyl rings on the both opposite sides of the manganese centre do not lie on a common plane. Although this is clearly due to the tetragonyl pyramid geommetry of the transition metal, there might also be a contribution from the close distance of tert-butyl groups on the ring. A space filling model constructed from van der Waals spheres shows that the atoms might experience some interaction. This could be confirmed with NCI analysis or Bond Critical Points calculation. It is also worth noting that one of the t-Bu groups is rotated out of the closest contact with the other group. This slight rotation (3o change of dihedral angle with respect to the ring plane) likely changes the unfavourable H-H contact to a slightly attractive interaction with carbon atoms.
In the following investigation, epoxidation of the following alkenes using Shi and Jacobsen catalysts were invesigated:
- styrene
- trans-beta-methyl styrene
- cis-beta-methyl styrene
The reaction with either catalyst proceeds via transition state where oxygen atom is added from one face of the alkene (i.e. syn addition), so the possible products of the epoxidation are:
- styrene: (R)-styrene oxide, (S)-styrene oxide
- trans-beta-methyl styrene: (R,R)-trans-beta-methyl styrene oxide, (S,S)-trans-beta-methyl styrene oxide
- cis-beta-methyl styrene: (R,S)-trans-beta-methyl styrene oxide, (S,R)-trans-beta-methyl styrene oxide
Shi catalysis transition states
Energies
Styrene: The free energy were used from precomputed transition structure (ref: mod:organic). For each expoxide theres for different 'sites' of attacks resulting in different enantimoers. The energies were found to the lowest energy transition state, the Arrhenius in terms of free energy of activation states:
In a kinetically controlled reaction, the concentrations are proportional to the rate constants of formation. Hence:
Below summarises the energies of each structures given its type of approach;
| Attack face Prochirality | Dioxirane Oxygen | type of approach | Structure | Free energy [a.u.] | Relative energy [kcal.mol] | Energy difference [J/mol] | CALCULATION | Relative Concentration [mol/dm3] |
|---|---|---|---|---|---|---|---|---|
| Re | Endo | -1303.92 | 6.80 | 28451.2 | DOI:10.6084/m9.figshare.829523 | 0.00 | ||
| Re | Exo | -1303.95 | 0.00 | 1 | DOI:10.6084/m9.figshare.829522 | 1.00 | ||
| Re | Bottom | Endo | -1303.92 | 8.98 | 37614.11 | DOI:10.6084/m9.figshare.830388 | 0.00 | |
| Re | Bottom | Exo | -1303.95 | 2.91 | 12259.12 | DOI:10.6084/m9.figshare.828552 | 0.0071 | |
| Si | Endo | -1303.74 | 0.30 | 1214.06 | DOI:10.6084/m9.figshare.829523 | 0.613 | ||
| Si | Exo | -1303.74 | 1.05 | 4435.00 | DOI:10.6084/m9.figshare.829522 | 0.1673 | ||
| Si | Bottom | Endo | -1303.71 | 5.19 | 21714.96 | DOI:10.6084/m9.figshare.830388 | 0.0002 | |
| Si | Bottom | Exo | -1303.71 | 4.89 | 20459.76 | DOI:10.6084/m9.figshare.828552 | 0.00029 |
Sum of Re concentrations is 1.0071 mol/dm3, and sum of Si concentrations is 0.78076 mol/dm3.
From the relative concentrations the enantiomeric excess can be calculated using the following equation:
, where Re and Si are the sum concentrations mentioned above.
ee(Styrene)= 12.8%, low enantiometric excess limiting its synthetic use.
Stillbene same calculations applied to styrene is repeated for stillbene.
+ Stilbene energies and concentrations| Attack face Prochirality | Dioxirane Oxygen | type of approach | Structure | Free energy [a.u.] | Relative energy [kcal.mol] | Energy difference [J/mol] | CALCULATION | Relative Concentration [mol/dm3] |
|---|---|---|---|---|---|---|---|---|
| Re | Endo | -1534.70 | 0.09 | 376.56 | DOI:10.6084/m9.figshare.829523 | 0.8591 | ||
| Re | Exo | -1534.70 | 0.00 | 0.00 | DOI:10.6084/m9.figshare.829522 | 1.00 | ||
| Re | Bottom | Endo | -1534.69 | 8.00 | 33555.68 | DOI:10.6084/m9.figshare.830388 | 0.00 | |
| Re | Bottom | Exo | -1534.69 | 7.67 | 32091.28 | DOI:10.6084/m9.figshare.828552 | 0.00 | |
| Si | Endo | -1534.69 | 10.40 | 43555.92 | DOI:10.6084/m9.figshare.829525 | 0.00 | ||
| Si | Exo | -1534.69 | 9.40 | 39435.00 | DOI:10.6084/m9.figshare.830389 | 0.00 | ||
| Si | Bottom | Endo | -1534.70 | 3.90 | 16714.96 | DOI:10.6084/m9.figshare.830390 | 0.0014 | |
| Si | Bottom | Exo | -1534.70 | 5.13 | 21459.76 | DOI:10.6084/m9.figshare.830389 | 0.0002 |
Sum of Re concentrations is 1.8591 mol/dm3, and sum of Si concentrations is 0.00016 mol/dm3.
ee(Stilbene)= 99.8%, very high (R,R) stereoselectivity
Jacobsen catalysis transition state
Energies
| Attack face Prochirality | type of approach | Free energy [a.u.] | Relative energy [kcal.mol] | Relative Concentration [mol/dm3] |
|---|---|---|---|---|
| S,R | Endo | -3383.26 | 0 | 1 |
| S,R | Exo | -3383.25 | 0.006 | 0.0015 |
| R,S | Endo | -3383.25 | 0.0084 | 0.0001 |
| R,S | Exo | -3383.25 | 0.0093 | 0.00 |
| Attack face Prochirality | type of approach | Free energy [a.u.] | Relative energy [kcal.mol] | Relative Concentration [mol/dm3] |
|---|---|---|---|---|
| S,S | Endo | -3383.26 | 0 | 1 |
| S,S | Exo | -3383.26 | 0.0046 | 0.0074 |
| R,R | Endo | -3383.25 | 0.0087 | 0.0001 |
| R,R | Exo | -3383.25 | 0.0081 | 0.0002 |
S,R sum = 1.0015; R,S= 0.0001
S,S= 1.0074; 0.0003
ee(cis)= 99.95%
ee(trans)=99.94%, Both really high epoxidation selectivity with Jacobsen catalyst.
Stimulation of specific rotation
| Epoxides | R,S-dihydronaphthalene oxides[9] | S,R-dihydronaphthalene oxides [10] | SS-trans-stilbene oxides [11] | R,R-trans-stilbene oxides [12] |
|---|---|---|---|---|
| Concentration [g/100ml] | 0.81 | 0.21 | 0.56 | 0.73 |
| Optical Rotation | 129o | -39o | -205.2o | 334.6o |
| Wavelength [nm] | 589 | 589 | 589 | 589 |
| Temperature | 20oC | 25oC | 20oC | 25oC |
| epoxides | R,R-trans-stilbene oxides DOI:10042/27501 | S,S-trans-stilbene oxidesDOI:10042/27523 | R,S-dihydronaphthalene oxides DOI:10042/27499 | S,R-dihydronaphthalene oxides DOI:10042/27524 |
|---|---|---|---|---|
| αd at 589 nm | 102.87o | -24.18o | 177.43o | -52.74o |
The predicted values calculated by the method mentioned above agrees with the literature values found with some extend of deviation tolerated. The sign of all predicted values perfectly match with the literature values. Therefore, the method introduced is reliable in calculating the optical rotation of those two epoxides.
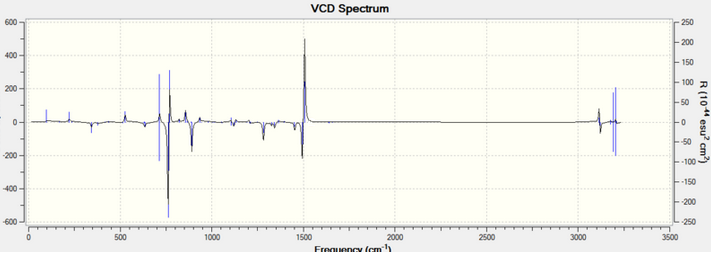
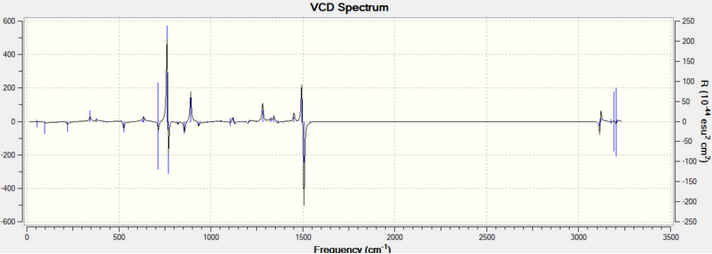
VCD
VCD spectra analysis is another method other than rotation analysis to assign absolute configurations via vibrational circular dichromism (VCD). The VCD spectas for R,R- and S,S-trans-stilbene oxide are shown below:
The two spectras are very similar due to their vibrational environments being opposite with respect to each other. The peaks at ~ 1500 and ~ 750 cm-1, point in opposite directions for both enantiomers. This allows configuration assignment to the enantiomers.
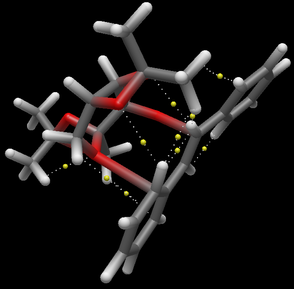
Non-Covalent interactions in active-site of transition state
NCI analysis was performed for the R,R-transitions of the Shi's catalyst promoted epoxidation of trans-stilbene, optimization and analysis was performed of Guassview.
Orbital |
The green regions indicates attractive interactions between the active catalyst and substrate via oxygen atom, the Van da Waals interaction between alkene and catalyst contribution to stabilisation of transition state. The substrate is able to self orientate to maximise attractive interactions, which in-turn will lower the energy of the activation state. Stabilisation of the transition state via attractive interactions will determine the stereoselectivity of the epoxidation.
QTAIM analysis
The QTAIM analysis was conducted to calculate the orientation of R,R-trans-stilbene oxide with respect to Shi's catalyst.
Suggestion of molecule for new investigation
My suggestion would be cis-R-(+)pulegone oxide which has a very high optical rotation at 854o in enthanol at 324 nm [13]
References
<references> [1] [3] [4] [5] [2] [6] [7] [8]
- ↑ 1.0 1.1 DOI:10.1002/ange.19340475202
- ↑ 2.0 2.1 Jerry March, Advanced organic chemistry, 4th edition, John Wiley & sons, New York, 1992.
- ↑ 3.0 3.1 DOI:10.1021/ja016622h
- ↑ 4.0 4.1 Goodman & Walsh. pp. 100-1
- ↑ 5.0 5.1 5.2 DOI:10.1021/ja00157a043
- ↑ 6.0 6.1 DOI:10.1016/S0040-4020(01)90678-7
- ↑ 7.0 7.1 7.2 DOI:10.1021/ja00398a003
- ↑ 8.0 8.1 DOI:10.1021/ja00274a016
- ↑ 9.0 9.1 Pedragosa-Moreau, S.; Archelas, A.; Furstoss, R. Tetrahedron 1996, 52, 4593
- ↑ 10.0 10.1 Lin, H.; Qiao, J.; Liu, Y.; Wu, Z.-L. Journal of Molecular Catalysis B: Enzymatic 2010, 67, 236
- ↑ 11.0 11.1 Niwa, T.; Nakada, M. Journal of the American Chemical Society 2012, 134, 13538
- ↑ 12.0 12.1 Wong, O. A.; Wang, B.; Zhao, M.-X.; Shi, Y. Journal of Organic Chemistry 2009, 74, 6335
- ↑ 13.0 13.1 Reusch; Johnson Journal of Organic Chemistry 1963, 28, 2557