Rep:Mod:yahoop
Part i - The hydrogenation of cyclopentadiene dimer





The dimerisation of cyclopentadiene proceeds via a π4s + π2s cycloaddition pericyclic reaction. Because the reaction pathway involves a cyclic transition state, it will be difficult to model with MM2 mechanics. MM2 mechanics has the limitation of approaching molecules with bonds in mind, and does not consider the orbital approach neccessary to understand pericyclic reactions.
| Stretch | Bend | Torsion | Intra-molecular VdW forces | 1,4-diaxial repulsion | Total | |
|---|---|---|---|---|---|---|
| exo diene | 5.45 | 86.07 | 32.07 | -5.93 | 17.65 | 133.38 |
| endo diene | 5.19 | 87.26 | 39.81 | -6.52 | 18.15 | 142.28 |
| ene 1 | 5.34 | 83.01 | 45.27 | -5.11 | 23.58 | 149.35 |
| ene 2 | 4.59 | 60.72 | 52.35 | -4.35 | 18.84 | 130.46 |
Whilst the exo product is predicted to be 8.9kj/mol more thermodynamically stable than the endo product, the endo product is formed exclusively. From this one can infer that the endo transition state is lower in energy than that of the exo, and the reaction has proceeded under kinetic control.
When the diene is hydrogenated, there is a chose of which C=C bond to reduce. The ene 1 and ene 2 products show differing intra-molecular VdW forces, 1,4-diaxial repulsion, stretch, torsion and bend. Which is to be expected as they are structurally distinct. The most important weighting factor in the relative thermodynamic stability of the two products is bend. The ene 2 is predicted to have 22.29 kj/mol less stretch than the ene 1. This represents the fact that losing a C=C bond in a six-membered ring leads to a far greater drop in ring strain than losing a C=C bond from a five-membered ring. This is because inclusion of a double bond in a six-membered ring forces a greater deviation from the ideal angle of the remaining C-C bonds. Overall this effect makes the ene 2 product more thermodynamically stable.
Since hydrogen is a very small molecule relative to the dimer steric issues are assumed to be negligible. Thus we expect hydrogenation to proceed under thermodynamic conditions, with the ene 2 being the major product.
Part ii - Stereoselective addition of nucleophiles to heteroaromatic ring systems

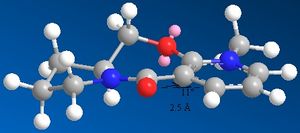

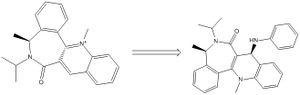
When a pronilol derivative is subjected to a grignard reagent, it undergoes addition to a certain plane of the molecule
The carbon shown in the scheme pointing up, has its orientation (in terms of being above or below the plane of the aromatic ring) mimicked by the carbonyl oxygen. This is demonstrated to the right.
Using Chemdraw 3D in conjunction with the MM2 force field program, the orientation of the carbonyl above the aromatic ring was found to be 12 degrees. The suspected reason for the delivery of the methyl onto the same plane of the molecule as the carbonyl, is due to a chelation of the carbonyl oxygen with the magnesium metal centre.
prolinol derivative 1 |
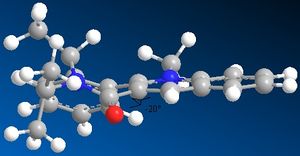
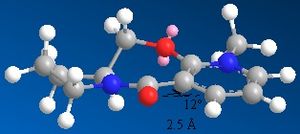
An alternative reaction of a nucleophile with a prolinol derivative is described to the left.
This time the carbonyl group is orientated 20 degrees away from the bicyclic aromatic system.In the case of the NH phenyl group acting as an electrophile,since now there is no metal oxygen coordination possible the preference is for the electrophile to add in on the plane molecule on the opposite side to the carbonyl group. This orientation minimises the electrostatic repulsions that would occur between the oxygen lone pairs and the the nitrogen lone pair.
prolinol derivative 2 |
Part iii - Stereochemistry governing taxol intermediate
An intermediate in the synthesis of taxol can has two possible conformations of a carbonyl group. The two possible conformations were modelled using Chem3D and then the energy minimised using the MM2 program.
interm. 1 |
The conformation shown above has the higher energy, 228.38 kj.mol-1
interm. 2 |
The second conformation shown above has energy 209.10 kj.mol-1 . This greater thermodynamic stability is attributed entirely to less ring strain, with the carbonyl pointing downward the bicyclic system is able to adopt a more planar, less strained conformation.
The alkene bond in this intermediate is found experimentally to react very slowly. This is due to the increase in ring strain experienced by the nine-membered ring as the tertiary carbon moves from an sp2 to an sp3 hybridisation state.
project molecule isomer 1 |
Part iv - Regioselectivity of electrophiles with chlorocarbene
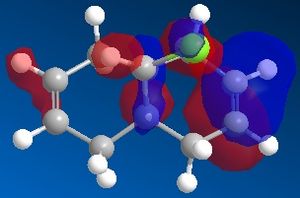
Chlorocarbene contains two alkene bonds , only one however is reactive with electrophiles. MM2 is inadequate in explaining this, therefore an orbital approach has to be adopted. The compound was modelled using the MOPAC/PM6 method, and the highest occupied molecular orbital was found, displayed to the right.
This is a clear demonstration of the increased electron desity that exists on the alkene cis to the chlorine. This model predicts that this will also be the more reactive alkene with respect to electrophilic attack.
Part v - Analysis of the stretching frequencies in chlorocarbene


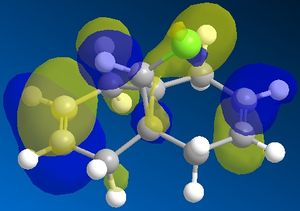
The electron density from the homo-1 of the alkene bond trans to C=O is donated into the σ* C-Cl. Weakening the bond and lowering the frequency of the C-Cl bond in the diene compared with that of the monoene.
