Rep:Mod:xz093
Introduction
Based on theories of quantum Chemistry, computational calculation methods can be used to find out the lowest energy geometry of a molecule. An optimization calculation can locate the local minimum point on the potential energy surface (PES) following the gradient and a frequency calculation which takes into account the curvature is used to confirm that the structure found is a real minimum. For a reaction, once the reactant and the product are all optimized to minimum, the reaction pathway can be studied by locating the transition state on PES. OPT=TS and OPT=QST2 are two of the methods for finding out the transition structure. The first one uses a guessed structure which is close to the transition structure and optimized to minimum oh the PES. The second one starts from the linked reactant and product and goes up in energy to find the maxima (TS). Intrinsic reaction coordinate (IRC) method can be used to proved that the transition states can lead to correct minima on PES. To calculate the energies, method/level of theory and basis set needs to be chosen. The method/level of theory is the Hamiltonian operator on the wavefunction and the basis set is the mathematical representation of the wavefunction. Semi-empirical, Hartree-Fock and Density Functional Theory are the methods applied in this experiment. The basis set used are 3-21G, the minimal basis set, and 6-31G*, which is more accurate.[1]
The Cope Rearrangement (tutorial)
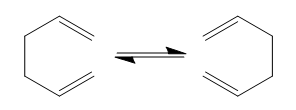
In this tutorial, the reaction pathways and transition structures of the Cope rearrangement of 1,5-hexadiene were studied. This reaction is a [3,3'] sigma tropic rearrangement that obeys the Woodward-Hoffmann rules, which states that for a thermally allowed pericyclic reaction, the total number of (4q+2)s and (4r)a components must be odd. [2]
The total energy of the reactant/product were investigated and two possible transition structures, chair and boat, were located on the potential energy surface (PES) using three different methods. The activation energies for both transition structures were calculated and compared with the experimental values. All of the molecules were build up by GaussView 5.0 and all of the calculations were done by Gaussian 09W.
Optimizing the Reactants and Products
Optimizing the 'anti' and 'gauche' 1,5-hexadiene
A 1,5-hexadiene molecule with 'anti' linkage (dihedral angle = 180°) was drawn and optimized to minimum using HF/3-21G level of theory. The .log file can be found here. .log file was opened and the total energy was record in summary under Results. The energy is -231.69097054 a.u.. The molecule has a symmetry and the point group is C1. This conformer is the anti4 in the Appendix 1. [1]
The total energy of the 'gauche' conformer was expected to be higher than 'anti' because of steric hindrance between the two vinyl systems. A 1,5-hexadiene molecule with 'gauche' linkage (dihedral angle = 60°) was drawn and optimized to minimum using same level of theory. The .log file can be found here.The total energy of the molecule is -231.69266121 a.u., which was lower than the optimized 'anti' conformer. This result was different from the expectation. The molecule has a symmetry and the point group is C1. This conformer is the gauche3 in the Appendix 1. [2]

Finding the lowest energy conformer
The lowest energy conformer of 1,5-hexadiene was expected to be the anti2 since the vinyl groups are farthest away from each other. However this was not the case for 1,5-hexadiene. The difference of distance between two vinyl hydrogen atoms in 'anti' and 'gauche' forms is very small. Therefore the steric effect is not significant. The gauche3 structure that has already been drawn and optimized in the last step is the lowest conformer of 1,5-hexadiene.[3] This is because there is attractive interaction between the C=C double bond and the vinyl proton. The filled π orbital can donating electron density to the empty σ* orbital and stabilize the molecule.[3]
(Re)optimizing the anti2 conformer
An anti2 1,5-hexadiene molecule was drawn and optimized using HF/3-21G level of method. The .log file can be found here. The molecule has a symmetry and the point group is Ci. The total energy of the molecule is -231.69253528 a.u. and the reference value given in the table in Appendix 1 is -231.69254 a.u..[4] They were almost the same. So the structure has been drawn correctly. The HF/3-21G optimized anti2 1,5-hexadiene molecule was reoptimized by DFT B3LYP/6-31G* level of theory. The .log file can be found here. The overall geometry changed very little from the previous HF/3-21G optimized structure. However, the total energy of the DFT B3LYP/6-31G* optimized structure is lower than the HF/3-21G optimized structure. This implies that the 6-31G* basis set is better than 3-21G in terms of finding the energy minima.
Frequency calculation on anti2 conformer
For comparison of the final total energies of the output file and the experimental values, frequency calculations need to be run. The DFT B3LYP/6-31G* optimized anti2 1,5-hexadiene molecule was performed a frequency calculation and all the vibrational frequencies were positive (i.e. no imaginary frequencies). The .log file can be found here. From the .log output file viewed in the visualizer, energies under different vibrational temperatures were noted down.
| sum of energies | in a.u. | in kcal/mol |
|---|---|---|
| electronic and zero-point energies | -234.469212 | -147131.683317 |
| electronic and thermal energies | -234.461856 | -147127.067357 |
| electronic and thermal enthalpies | -234.460912 | -147126.474988 |
| electronic and thermal free energies | -234.500821 | -147151.518269 |
Optimizing the 'chair' and 'boat' Transition Structures
It is proposed that the Cope rearrangement reaction can take place via two different transition structures, regarding as 'chair' and 'boat'. Both transition structures were located and optimized but with different methods.
Optimizing the 'chair' transition structure (method 1)
The 'chair' transition structure was located and optimized using the method of calculating the force constant. Two identical allyl fragments were drawn and optimized using the HF/3-21G level of theory. The .log file can be found here. They were moved into an orientation that was close to the 'chair' transition structure with a distance of approximately 2.2 Å apart. This is a guess transition structure. Opt+Freq calculations were carried out with optimization to TS (Berny) and calculated the force constant once. Opt=NoEigen was put in as additional keywords. The output molecule had only one negative vibration frequencies at -817.90 cm-1 and the rest were positive frequencies. The .log file can be found here. The animation of this imaginary frequency was identified to be the Cope rearrangement as shown below. The RMS Gradient Norm of the molecule was 0.00001426 a.u., which was almost zero. These factors confirmed that this was the correct transition structure.
'chair' transition state (method 1) |
The imaginary frequency represents a negative force constant k. Since for a 1D quantum harmonic oscillator, k is the second derivative of the energy (i.e. curvature). It would be negative at the energy maxima, where a transition state is on the potential energy surface. Therefore the vibrational frequency would be negative from the equation below.
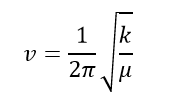
Optimizing the 'chair' transition structure (method 2)
The 'chair' transition structure was optimized using the frozen coordinate method. On the guess structure, Redundant Coord Editor was used to freeze the four carbon atoms that form/break bonds respectively and left the rest of the molecule free for minimization. Here optimization to minimum was operated instead of optimization to TS (Berny). Force constant was not calculated. The output molecule showed a fixed distance of 2.2 Å between the bond form/break carbon atoms. Redundant Coord Editor was used again to unfreeze the carbon atoms and this time optimized to TS (Berny). Again, force constant was not calculated. The output molecule had only one negative vibration frequencies at -817.88 cm-1 and the rest were positive frequencies. The .log file can be found here. The animation of this imaginary frequency was identified to be the Cope rearrangement as shown below. The RMS Gradient Norm of the molecule was 0.00002117 a.u., which was almost zero. Again these factors confirmed that this was the correct transition state.
'chair' transition state (method 2) |
The bond form/break bond lengths of the transition structures generated by the two methods were very similar with the one from method 2 slightly higher.
| Bond break/form C-C bond lengths (Å) | |
|---|---|
| method 1 | 2.02064 |
| method 2 | 2.02071 |
Optimizing the 'boat' transition structure
The 'boat' transition structure was located and optimized using the TS(QST2) method. In this method, the reactant and product were drawn out and given the same number to each atom. The calculation can work out the transition structure based on the numbering and orientation of the reactants and products. The DFT B3LYP/6-31G* optimized anti2 1,5-hexadiene were used as both reactant and product and the two molecules were modified as below. It illustrated that from reactant to product the bond between C3 and C4 broke and between C1 and C6 formed. The double bond moved from between C1,C2 and C5,C6 to C2,C3 and C4,C5. The input molecules looked like this.
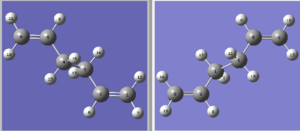
However in this case, the job failed. The .log file can be found here. The TS(QTS2) method was not able to locate the correct transition state from this arrangement of reactant and product because it is too deviated from the actual 'boat' transition state structure. So the input molecules were modified by changing the central C-C-C-C dihedral angle to 100° and the inside C-C-C angle to 100°. The numbering of each atom were kept unchanged. The molecules now looked like this.
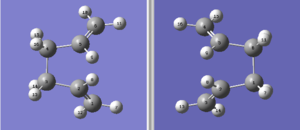
This time the input molecules were close enough to the 'boat' transition structure and it was successfully located. The .log file can be found here. Again a negative frequency was shown at -840.05 cm-1 and the corresponding animation (below) indicated the Cope rearrangement reaction.
'boat' transition state |
This exercise showed that although the TS (QTS2) method was able to directly calculated the transition structure from just the reactant and product, it was only valid when the input reactant and product were modified in a way very close to the actual structure of the desired transition state. Otherwise the transition structure could not be achieved.
Intrinsic reaction coordinate (IRC) method
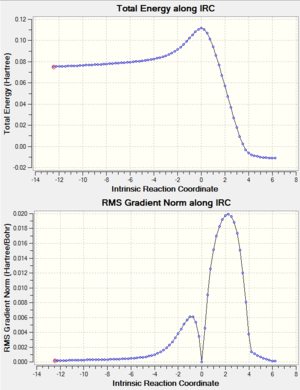
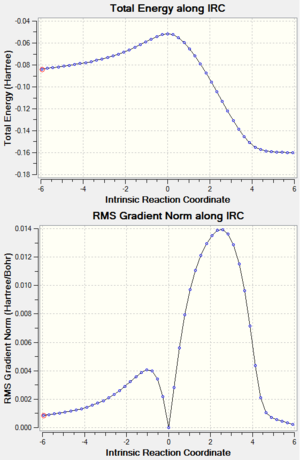
Now that the 'chair' and 'boat' transition structures were found out. Yet the conformer of 1,5-hexadiene that each transition structures connect to was difficult to predict. The IRC method was used to locate the local minimum from the transition structure on the potential energy surface. This calculation can also prove that the transition structure was correctly located. Here this method was applied to the HF/3-21G optimized 'chair' transition structure. For calculation set up, only forward direction was chosen because the reaction coordinate was symmetrical. The reactant and product should have the same structure and therefore the same energy. Force constant calculation was chosen to be always and the points generated along the IRC path was set to be 50. The results were as shown below. The .log file can be found here
ANIMATION ADDED

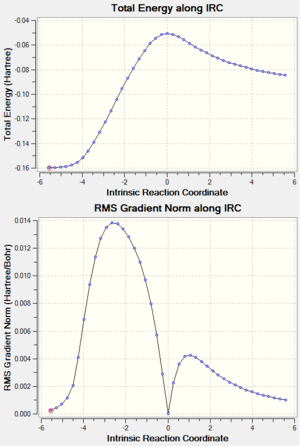
Both the total energy in summary and the IRC plot showed that the minimum energy geometry had not been reached. (The total energy of gauche3 conformer is -231.69266 a.u.).[5] Therefore IRC method was improved to locate a further minimized structure. The last point on IRC plot, which was the minimum from last calculation, was taken to run a normal minimization. The .log file can be found here. The output geometry had a lower total energy than the input as shown in the table. Other possible improvement methods include increasing of the point on the IRC path and calculating the force constant on every step.
| total energy in a.u. | |
|---|---|
| input geometry | -231.69158 |
| output geometry | -231.69167 |
Activation energies
The activation energies of the reaction for both 'chair' and 'boat' transition structures were calculated and compared. Both 'chair' and 'boat' transition structures were reoptimized then calculated frequencies using DFT B3LYP/6-31G* level of theory from the previously HF/3-21G optimized structures. The .log files can be found here and The .log file can be found here. As a result, the geometries of reactant and transition structure were very similar for each level of theory,showing that 3-21G basis set was good enough to get the correct geometry of the transition structure. However the total energy difference was large. The activation energies were calculated at 0K and 298.15K from the raw data (in a.u.) collected in the .log file for both transition structures and compared to the experimental values. [6] 1 hartree = 627.503 kcal/mol. [4]
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6.31G* | Experimental values | |
|---|---|---|---|---|---|
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE in kcal/mol (Chair TS) | 38.4 | 37.3 | 34.1 | 33.2 | 33.5 ± 0.5 |
| ΔE in kcal/mol (Boat TS) | 48.2 | 47.4 | 42.0 | 41.3 | 44.7 ± 2.0 |
The comparison of results showed that the activation energies calculated from DFT B3LYP/6-31G* is closer to the experimental values the ones from HF/3-21G. The values were almost in the range of the experimental data. This indicated that the Density Functional Theory (DFT) method is more accurate than the Hartree Fock (HF) method. This is because the DFT method includes the exchange-correlation term, which shows the correction to the kinetic energy due to interacting nature of electrons, as well as all of the nonclassic corrections to the repulsion energy between electrons. [5]
The Diels-Alder Cycloaddition
The Diels-Alder Cycloaddition is another type of pericyclic reaction in which an electron-rich cis diene react with an electron-deficient dienophile to form two new σ C-C bonds. In this section, the transition structures and reaction mechanism of a prototypical reaction and a reaction with substituted diene and dienophile were found out and investigated.
Part 1. cis-butadiene + ethlyene
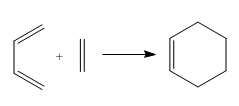
The reaction of cis butadiene and ethlyene was studied as a prototype cycloaddition reaction. The reaction obeys the Woodward-Hoffmann rules and is thermally allowed.
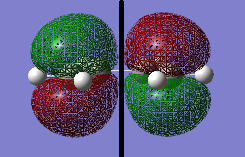
A cis butadiene and the molecule was optimized by Semi-empirical AM 1 .log file. and then DFT B3LYP/6-31G* level of theory .log file. The MOs of the molecule were visualized. The HOMO of cis butadiene is anti-symmetric (i.e. a) and the LUMO is symmetric (s) with respect to the plane (indicated by the black line in the middle).
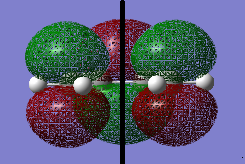
It is stated that the reaction has an envelop shape transition structure for maximizing the overlap between π systems of the cis butadiene and the π orbital of the ethylene. A guess transition structure with a distance of 2.2 Å between bond break/form C-C atoms was drawn and optimized by the method 1 (TS Berny) in the Cope Rearrangement tutorial on Semi-empirical AM 1 level. .log file. The output structure was confirmed to be the transition state because (i) it had one and only one negative (imaginary) vibrational frequency and the animation showed the bond formation. (ii) the RMS Gradient Norm was nearly zero. IRC was run on the Semi-empirical AM 1 level to follow the minimum energy path from the transition state. .log file. The formation of the two new σ C-C bonds are synchronous. This was to compare with the lowest positive vibrational frequency in which the two bonds formed asynchronously.
Vibration |
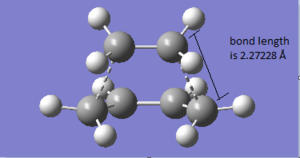
The geometry of the transition structure is shown below. The bond length of the partly formed C-C σ bond is 2.27228 Å. From the literature, the typical sp3 and sp2 C-C bond length are 1.54 Å and 1.46 Å[6] and the van der Waal radius of the carbon atom is 1.70 Å[7]. The bond length of partly formed C-C is longer than sp3 and sp2 bond length and even longer than the van der Waal radius of the carbon atom. It can be concluded that at this stage the two substrates are not forming bonds and only having interactions weaker than van der Waal force.
The MOs of the transition structure were visualized. Both of the HOMO and LUMO were symmetric (s) with respected to the plane (black line).
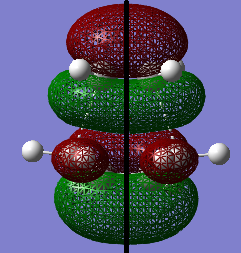
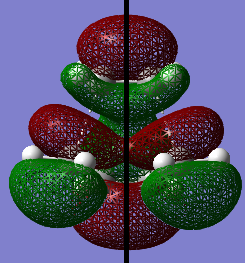
The HOMO at the transition structure is symmetric (s). It is formed by the HOMO of cis butadiene and the LUMO of ethylene because the two MOs have the same polarity, therefore they are able to interact. Also the energy difference between these two MOs is smaller than the energy between the LUMO of butadiene and HOMO of ethylene. The smaller energy gap between MOs also cause better orbital overlap. This ensures that there is a significant overlap density between the two MOs. Therefore the reaction is allowed.
| cis butadiene | ethylene | |
|---|---|---|
| LUMO energy | -0.03012 | 0.01881 |
| HOMO energy | -0.22735 | -0.26664 |
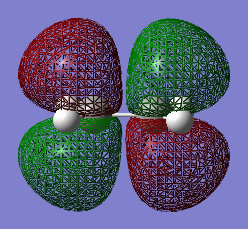
Part 2. cyclohexa-1,3-diene + maleic anhydride
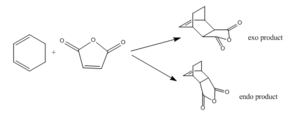
In this reaction, the diene is cyclohexa-1,3-diene and the dienophile is maleic anhydride. The regioselectivity of the reaction was studied. It is proposed that the endo product dominates under kinetic control.
The two reactants were drawn and optimized using Semi-empirical AM 1 (cyclohexadiene .log file and maleic anhydride .log file) and then DFT B3LYP/6-31G* level of theory (cyclohexadiene .log file and maleic anhydride .log file). The MOs were visualized. The LUMO of cyclohexa-1,3-diene was asymmetric (a) and HOMO was symmetric (s) with respect to the plane. The LOMO of maleic anhydride is asymmetric (a) and HOMO is symmetric (s) with respect to the plane. The dienophile in this case is oxygen-substituted, which is an electronegative heteroatom and it has two carbonyl as electron-withdrawing groups. Therefore the LUMO and HOMO would be lower in energy.[2] The reaction occurs between the HOMO of cyclohexa-1,3-diene and the LUMO of the maleic anhydride due to a small energy gap and better overlap.
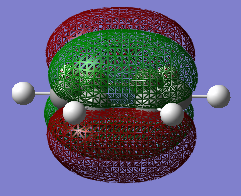
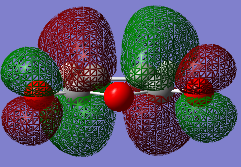
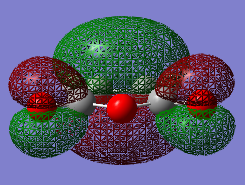
-
Caption 1
-
Caption 2
The optimized fragments were then copied and pasted into the same window and rearranged to two different structures to form the guessed endo and exo transition structures. The dihedral angles of the planar fragments were adjusted to more like each of the transition structures. Then method 1 (TS=Berny) from previously was used to locate both of the transition structures, firstly Semi-empirical AM1 (endo TS AM1.log file and exo TS AM1.log file) then DFT B3LYP/6-31G* (endo TS 631G.log file and exo TS 631G.log file). The imaginary vibrational frequency of endo TS was at -806.20 cm-1 and of exo TS was at -812.46 cm-1. IRC were run for both 3-21G optimized transition structures on the Semi-empirical AM1 level. (endo IRC.log file and exo IRC.log file). Higher levels such as DFT B3LYP/6-31G* can be run using HPC for more accurate results. The energies and geometries of the two transition structures were compared and studied.
endo transition state |
The endo product that was from the end point of IRC was taken to run a optimization to minimum on DFT B3LYP/6-31G* level. .log file The bond length of the newly formed C-C σ bonds are 1.55892 Å, which is shorter than the van der Waals radii of carbon (1.70 Å) and similar to the sp3 C-C bond length (1.54 Å), suggesting bonds formation.
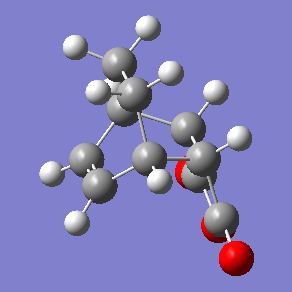
exo transition state |
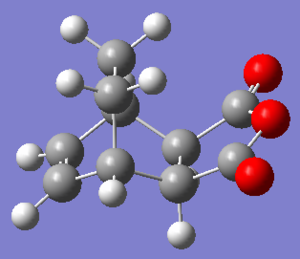
The exo product that was from the starting point of IRC was taken to run a optimization to minimum on DFT B3LYP/6-31G* level. .log file The bond length of the newly formed C-C σ bonds are 1.56200 Å, which is shorter than the van der Waals radii of carbon (1.70 Å) and similar to the sp3 C-C bond length (1.54 Å), suggesting bonds formation.
To study why the endo product dominates under kinetic control of the reaction, the endo and exo transition states were further investigated. Frequency calculations were run on the two transition structures on DFT B3LYP/6-31G* level. The thermochemistry data of the two were noted and the activation energies for both cases were calculated.
| B3LYP/6-31G* | electronic energy in a.u. | sum of electronic energy and zero-point energy in a.u. | sum of electronic energy and thermal energy in a.u. |
|---|---|---|---|
| cyclohexadiene | -232.24866 | -233.17583 | -233.17131 |
| maleic anhydride | -379.28483 | -379.22820 | -379.22337 |
| endo TS | -612.68340 | -612.50214 | -612.49179 |
| exo TS | -612.67931 | -612.49801 | -612.48766 |
| at 0 K | at 298.15 K | |
|---|---|---|
| ΔE of endo TS in kcal/mol | 61.6 | 60.9 |
| ΔE of exo TS in kcal/mol | 59.0 | 58.3 |
The energy of endo transition structure is lower than exo, so the activation energy of endo is lower than exo. Therefore the endo form would form more quickly than exo under kinetically controlled reaction condition. The results comply with the proposed statement. The energy difference between the two transition structures can be explained by their geometries and frontier orbital interactions.
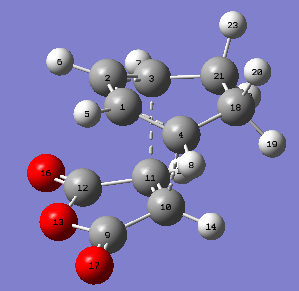
| carbon atoms | bond length in Å |
|---|---|
| C4-C10 | 2.26848 |
| C3-C11 | 2.26848 |
| C1-C4 | 1.39143 |
| C2-C3 | 1.39143 |
| C4-C18 | 1.51498 |
| C3-C21 | 1.51498 |
| C18-C21 | 1.55843 |
| C9-C10 | 1.47948 |
| C11-C12 | 1.47948 |
| C10-C11 | 1.39397 |
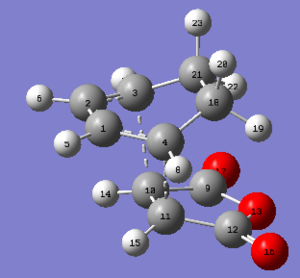
| carbons atoms | bond length in Å |
|---|---|
| C4-C11 | 2.29057 |
| C3-C10 | 2.29057 |
| C1-C4 | 1.39139 |
| C2-C3 | 1.39139 |
| C4-C18 | 1.51453 |
| C3-C21 | 1.51453 |
| C18-C21 | 1.55839 |
| C9-C10 | 1.47942 |
| C11-C12 | 1.47942 |
| C10-C11 | 1.39789 |
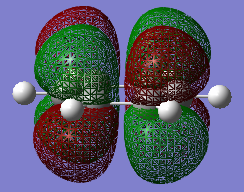
The C-C bond lengths within each fragment are similar for both cases. Yet the through space C-C bond lengths are more different. The exo form could be more strained because the partly formed C-C σ bond lengths for exo form are longer then endo. So the steric hinderance between the two fragments in exo form could be more significant. The C-C through space distance between the -(C=O)-O-(C=O)- fragment of the maleic anhydride and the carbon atoms of the 'opposite' -CH2-CH2- for the exo is 2.94575 Å, which is further apart than the corresponding distance for the endo form which is 2.89225 Å. However the endo form has a lower energy and is favored in kinetically controlled reaction. Therefore this leads to the fact that there is secondary orbital overlap present in the endo form that counteract and override the steric repulsion of the closer distance between -(C=O)-O-(C=O)- and the opposite -CH=CH-. This interaction does not exist in exo form. It can be explained by looking at the HOMO of these two transition structures.
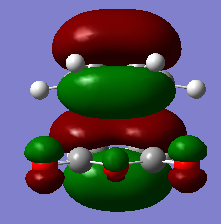
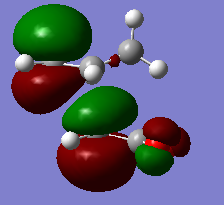
There are two kinds of interactions between the frontier orbitals of the two fragments in the endo form, which are primary orbital interaction and the secondary orbital interaction. The primary orbital interaction is between the p orbitals of the two bond-forming carbon atoms on the diene and the p orbitals of the two bond-forming carbon atoms on the dienophile. This in phase interactions cause new C-C bonds formation. It is present in both endo and exo transition structures. The secondary orbital interaction is between the p orbitals of the two nearby carbon atoms in the diene and p orbitals on the carbon atoms of the C=O groups of the dienophile. This is a through space interaction and it is formed by the overlapping of the lower part of the p orbital of the two carbon atoms on the diene and the upper part of the C=O p orbital because they have the same polarity. On the lab manual, it suggests to visualize the effect on the HOMO of transition state. Yet the effect is not recognizable in HOMO, but in the MO below HOMO, as shown in the image. Since that is also a filled MO, it can still stabilize the endo transition structure. This secondary orbital interaction does not lead to bond formation, but would lower the energy of the endo transition structure.[8] Since it does not exist in the exo form due to opposite prientation of the dienophile, the endo form has a lower energy and would form more quickly in the kinetically controlled reaction.
The electrostatic solvent effect is not considered in this experiment. The geometry of transition structures may be modified in solution. The solvent polarity may influence the endo/exo selectivity for Diels-Alder reactions.[9]
Conclusion
In this computational experiment, the reaction pathways and transition structures of two types of pericyclic reactions were studied. For the Cope rearranegment reaction, three different methods were used to find out the transition structures on PES. Minimal basis set (3-21G) was proven to be sufficient to find out the correct geometry and higher level of basis set (e.g. 6-31G*) was required for more accurate energy calculation. A prototype reaction with butadiene and ethylene was studied and the transition structure was computed. The regioselectivity of Diels-Alder cycloaddition of cyclohexa-1,3-diene and maleic anhydride was investigated using the frontier orbitals. The endo transition state was proven to be favored under kinetic control due to presence of the secondary orbital interaction.
For improvement of the experiment, the transition structures of Diels-Alder reactions can be located using other methods such as Frozen Coordinate and QST2 and compare the results with TS(Berny).
References
- ↑ Bearpark, M. Quantum Mechanics 3 lecture notes
- ↑ 2.0 2.1 Gibson. S. Pericyclic Reactions lecture notes
- ↑ Gung, B. W; Zhu, Z; Fouch, R. A. J. Am. Chem. SOC., 1995, 117, 6.
- ↑ Energy Unit Convertion Table. Available from: http://users.mccammon.ucsd.edu/~dzhang/energy-unit-conv-table.html [Accessed 17th December 2015]
- ↑ Sousa, S. F; Fernandes, P. A; Ramos, M. J. J. Phys. Chem. A 2007, 111, 10439-10452
- ↑ Zhou, G. M. Fundamentals of Structural Chemistry. World Scientific Pub Co Inc; 1993. Available from: https://books.google.com/books?id=IzDmZT6LKBMC&pg=PP5&lpg=PP5&dq=Fundamentals+of+Structural+Chemistry&source=bl&ots=qPLpLGnI4s&sig=2fLsNMwpxrMhxbr0kEJc9wG7w0U&hl=en&sa=X&ved=0ahUKEwiJp42BnN7JAhXDtBQKHVCPBUwQ6AEIMTAD#v=onepage&q=Fundamentals%20of%20Structural%20Chemistry&f=false [Accessed 17th December 2015].
- ↑ Mantina, M; Chamberlin, A. C; Valero, R; Cramer, C. J; Truhlar, D. G. J. Phys. Chem. A 2009, 113, 5806–5812
- ↑ Ian F. Molecular Orbitals and Organic Chemical Reactions. John Wiley & Sons, Ltd; 2010. Available from: http://jpkc.huanghuai.edu.cn/include/htmleditor/uploadfile/20130309150247611003.pdf [Accessed 17th December 2015].
- ↑ Ruiz-Mpez, M. F; Assfeld, X; Garcia, J. A; Mayoral; Salvatella, L. J. Am. Chem. Sot. 1993,115, 878&8787


