Rep:Mod:xy307
Third Year Computational Lab Module 1:
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
Objectives of this module of the course:
Computers can be widely used in modelling many aspects of organic structures and reactivities today. These modellings not only provide the rationalities of the outcomes of the reactions, but also predict possible modifications or even new reactions. The module contains some basic information of molecular modelling and selects several examples to illustrate the ideas. It ends with an open mini-project which relates to common synthetic chemistry labs. The module includes the following aspects:
- To use molecular mechanics to predict the geometry and regioselectivity of:
- The hydrogenation of cyclopentadiene dimer
- The stereochemistry of nucleophilic addition to two different pyridinium.
- The conformation/atropisomerism of a large ring ketone intermediate in one synthesis of the anti-cancer drug Taxol
- To use semi-empirical and DFT molecular orbital theory to investigate:
- The origins of the regioselectivity of the electrophilic carbenylation of a chloro-substituted bicyclic diene,
- The use of DFT molecular orbital theory to investigate Neighbouring group participation on the C-Cl and/or C=C stretching frequency of the above bicyclic diene
- concluding with a Mini-project investigating spectroscopic simulation in an organic molecule.
- To gain familiarity with the use of a institutional digital repository
- To perform searches of the literature for each topic in order to cite in your final report any relevant references to each experiment as appropriate.
- To present the results in the form of a Wiki page.
The Hydrogenation of Cyclopentadiene Dimer
The purpose of this part is to determine the product of cyclopentadiene dimerisation and the hydrogenation of dimer. The MM2 method in ChemDraw 3D is used to optimize the emergy and geometry. By comparing with the energy difference, the determinig factor(thermodynamics or kinetics) in dimerisation is decided, and the hydrogenation product of the dimer can be found out on a thermodynamic basis.
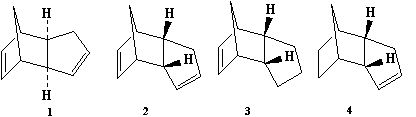
Cyclopentadiene dimerises to produce specifically the endo dimer 2 rather than the exo dimer 1. Hydrogenation of this dimer proceeds to give initially one of the dihydro derivatives 3 or 4. Only after prolonged hydrogenation is the tetrahydro derivative formed.
The optimized conformations for both 1 and 2 and their corresponded are shown below, so do their 3D structures. The modelling at MM2 level shows that molecule 1 has a total energy of 31.8834kcal/mol while 2 has an energy of 34.0153kcal/mol. The main contribution of the energy difference comes from the torsion. (7.6715 vs 9.5039kcal/mol) The result indicates 1 is thermodynamically more stable than 2. However, experiment shows the endo dimer 2 is the major product, which means the reaction is kinetically rather than thermodynamically controlled.
| Table 1 - MM2 Optimization | ||||
|---|---|---|---|---|
| Molecule | 1 | 2 | 3 | 4 |
| Parameter | Energy / kcal mol-1 | |||
| Stretch | 1.2923 | 1.2454 | 1.2241 | 1.0963 |
| Bend | 20.5870 | 20.8603 | 18.8675 | 14.5074 |
| Stretch-bend | -0.8413 | -0.8320 | -0.7567 | -0.5493 |
| Torsion | 7.6715 | 9.5063 | 12.2463 | 12.4972 |
| Non-1,4 VDW | -1.4358 | -1.5038 | -1.5627 | -1.0507 |
| 1,4 VDW | 4.2320 | 4.3012 | 5.7518 | 4.5124 |
| Dipole/Dipole | 0.3778 | 0.4448 | 0.1631 | 0.1407 |
| Total | 31.8834 | 34.0153 | 35.9337 | 31.1540 |
The result table on the left shows that molecule 4 is about 4.8kcal/mol lower in energy than molecule 3, which means 4 is the thermodynamic product of hydrogenation. Comparing with 3, the main energy difference contributes from the Bend term (~4.4kcal/mol). It suggests that in molecule 3 the double bond feels a stronger strain than in 4. The effect probably come fron the bridging carbon atom above the 6-member ring. The atom leads the ring deviates from normal boat conformation and the the two bonds connected to the double bond compress. As a consequence, the angle between double-single C-C bond is much less than usually sp2 bond angle-120 degree. For 3, 5-member ring is not restricted by other bond or group, so it is conformational flexible at some extent to reduce this kind of compression. The 1,4-VDW difference is 1.2kcal/mol in favour of 3, which is also due to the ring restriction and compromises some of the bend strain. The differences in stretch, stretch-bend, and non-1,4 VDW energies are tiny and don’t contribute significantly to the overall energy difference.
Stereochemistry of Nucleophilic Addition to a Pyridium Ring (NAD+ Analogues)
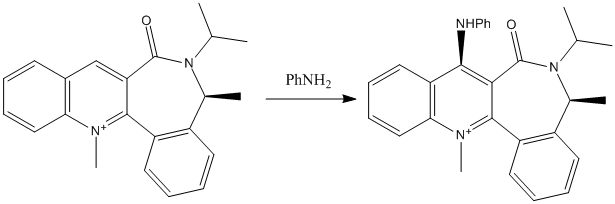
The purpose of this section is to rationalize two stereospecific reactions about nucleophilic addition to a pyridiumring. One is the addition of MeMgI to molecule 5, which is an optical derivative of prolinol; the product is exclusively molecule 6. The other is the addition of phenylamine to molecule 7, which also attacked the ring at 4-position and gives molecule 8. Another optimizing method, MMFF94 is is used here to determine the geometry and minimize the energy of the investigated molecules.

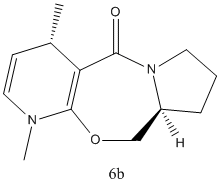
The addition product is exclusively 6a, rather than 6b, which is in contrast with the thermodynamic data.(At MM2 level, 6a is 37.49lcal/mol and 6b is 37.42kcal/mol. The product should be a mixture of both)
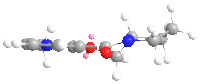
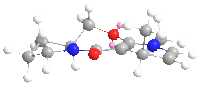
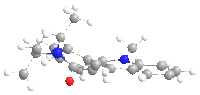
From the geometry optimized by MMFF94, it can seen that the carbonyl group is slightly above the aromatic ring, while in product 6a the methyl group is also above the ring but in 6b it is below the ring. The chelation between the magnesium of the nucleophile and the carbonyl oxygen favours addition at the same side of the plane, so 6a is the exclusive product.
From the optimization at MMFF94 level, it is obviuos that the reaction site is highly hindered by the methyl groups. On the other side, the coming group contains a large phenyl group. Consequently, the only way for the reaction to take place is the addition from the less hindered site, which results in exclusive produce 8. The product is a thermodynamically unstable molecule since it as an energy of more than 90kcal/mol form MMFF94 level optimization.
Stereochemistry and Reactivity of and Intermediate in the Synthesis of Taxol
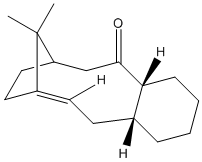
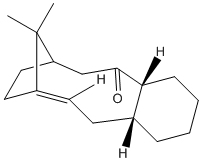
| Comparison of 9 and 10 | ||||
|---|---|---|---|---|
| Molecule | 9 | 10 | ||
| Parameter | Energy / kcal mol-1 | |||
| MM2 | 49.88 | 55.90 | ||
| MMFF94 | 66.39 | 77.09 | ||
Both MM2 and MMFF94 molecular mechanics calculation show molecule 10 has a higher energy than 9. The most important factor in the example is the retension of conformation the C=C and C=O bond. These bonds includes a sp2 hybridized carbon and requires a trigonal planar conformation at the carbon centre. In molecule 9, the conformation is easily achieved, but in molecule 10, the confromation require stronngly distortion of 9-member ring in the middle so the 5-memember ring on the left is highly compressed. The bend of the ring contrbutes most of the energy difference. On the other side, the methyl group hydrogen on the bridged carbon could interact with oxygen lone pair and the stereoelectronic effect also favours 9.
Both 9 and 10 are examples of hyperstable olefins. They are quite stable because of the reluctance to undergo hydrogenation across the double bond, which is in contrary to the bond theory convention. Furthermore, the hyperstable alkenes are thermodynamically more stable than their hydrogentated counterparts. The odd property is caused by the increasing strain after hydrogenated. The new bond formation cannot compensate the strain exerted by the transformation from sp2 carbons to sp3 carbons, which will destabilise the already conformationally restricted system.
Modelling Using Semi-Empirical Molecular Orbital Theory
In this section we can see the weakness of traditional purely classical treatment of a molecule and will move to a quantum method. We will consider the electrons in the molecule and how they affect bond, reactivity and spectroscopy properties.
Regioselective Addition of Dichlorocarbene
Part 1
Molecule 12 is a diene which can illustrate orbital control of reactivity when react with electrophilic reagents such as carbene or peracid. To model such a reaction, we need to predict the geometry, energy and molecular orbital by quantum mechanical methods rather than molecular mechanical methods.
The MM2 method is used first to clean up the geometry, than followed by the MOPAC/PM6 method, which is used to show molecular orbitals and energies. Four molecular orbitals are shown below, which are HOMO, LUMO, LUMO+1 and LUNO+2.
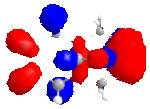 |
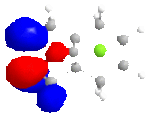 |
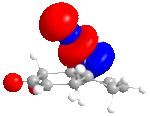 |
 |
In the reactions, the dialkene act as an nucleophile. The HOMO of it will react with LUMO of an electrophile. It is obvious that the double bound with more electron density at HOMO reacts better. From the pictures above we can see this is the bond at Cl side. As a result, if we don't consider kinetics or steric effect, this bond is in favour of electrophilic attack in the reaction.
Part 2
The purpose of this section is to compare the influence on the C-Cl bond when a double bond anti to the C-Cl is hydrogenated to a single bond. First, we need choose B3LYP/6-31G(d,p) Gaussian geometry optimization and frequency calculation;then we can compare the results and check the difference in the stretches.

| Bond Type | Dialkene
Stretching Frequency/cm-1 |
Monoalkene
Stretching Frequency/cm-1 |
|---|---|---|
| C=C, near Cl side | 1757.44 | 1758.07 |
| C=C | 1737.05 | - |
| C-Cl | 770.82 | 774.98 |
In the table we can see molecule 13(the hydrogenation product) lacks an absorption at about 1730-1760cm-1, which is due to the loss of a C=C bond. Comparing with the two double bonds in dialkene, the one near Cl side has higher frequency than the other which is broken in hydrogenation first.It is in accordance with the previous molecular orbital diagrams.
The slightly weaker C-Cl bond in the dialkene can be explained with MO diagrams as well. The alkene away from Cl can donate electron density into the LUMO+1 C-Cl antibonding orbital. After hydrogenation, the single bond at that position cannot make contribution to the antibonding since the electron density is stably located on the bond.
Mini Project: High-level Geometry Optimization and Spectroscopic Characterization of a Cycloaddition Step in the Synthesis of 2-epi-Pumiliotoxin C
Intramolecular Diels-Alder cycloaddition of N-substituted oxazolone triene I allows direct entry to the functionalized octahydroquinoline II. Further manipulation of this framework by stereo- and regioselective introduction of the 5-methyl substituent, followed by excision of the carbamate, yields (()-2-epi-pumiliotoxin C.
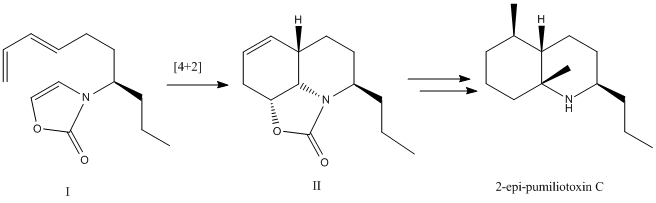
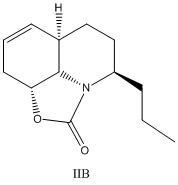
The cycloaddtion step is stereoselective and results in a mixture of II and IIB in a ratio of 3.7:1
Predicting 13C NMR Spectra of Compounds
The molecules are optimized by ChemDraw 3D using MM2 molecular mechanics first. Then the result structure is turned into a Gaussian input file, with DFT=mpw1pw91 method and on 6-31G(d,p) basis set. After optimizing the input file, we get a new optimized geometry and submit the output again for NMR analysis. The result can be illustrated with GaussView and compared to the literature.
The calculated values of 13C NMR fit the experimental values very well, especially when the chemical shift is lower than 100ppm. However, it is noticable when the chemical shift is greater than ~150ppm, the calculated values are normally ~20-30ppm greater.
Major Product, (31S,4R,6aS,9aR)-4-propyl-4,5,6,6a,9,9a hexahydrooxazolo[5,4,3-ij]quinolin-2(31H)-one,II
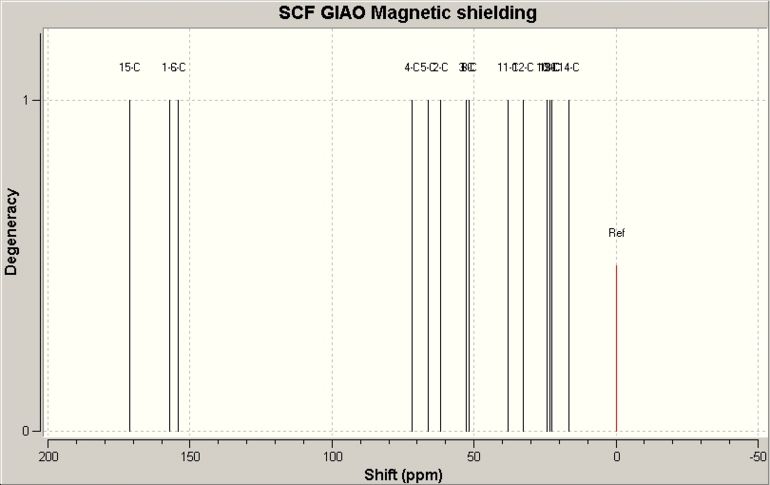
| Carbon | Calculated
Chemical Shift/ppm |
Reference
Chemical Shift/ppm |
|---|---|---|
| 14 | 16.2 | 19.4 |
| 10 | 22.3 | 22.6 |
| 13 | 23.1 | 23.3 |
| 9 | 23.8 | 27.6 |
| 3 | 32.4 | 30.9 |
| 8 | 37.8 | 31.8 |
| 2 | 61.5 | 48.5 |
| 5 | 65.7 | 51.9 |
| 4 | 71.5 | 72.4 |
| 6 | 153.9 | 126.7 |
| 1 | 156.8 | 131.4 |
| 15 | 170.7 | 157.2 |
Minor Product: (()-(31S,4R,6aR,9aR)-4-propyl-4,5,6,6a,9,9a-hexahydrooxazolo[5,4,3-ij]quinolin 2(31H)-one, IIB

| Carbon | Calculated
Chemical Shift/ppm |
Reference
Chemical Shift/ppm |
|---|---|---|
| 14 | 17.0 | 19.5 |
| 13 | 24.7 | 23.4 |
| 10 | 28.2 | 28.4 |
| 9 | 30.1 | 28.7 |
| 3 | 31.9 | - |
| 2 | 33.2 | 33.2 |
| 5 | 37.9 | 37.0 |
| 11 | 41.4 | - |
| 12 | 43.1 | - |
| 8 | 53.6 | 49.8 |
| 4 | 60.0 | 57.2 |
| 1 | 127.3 | 125.4 |
| 6 | 135.5 | 132.0 |
| 15 | 173.5 | 157.6 |
The calculated 13C NMR spectra are in highly consistence with
Prediction IR Spectra of compounds
The IR spectra are caculated by DFT method at b3lyp/6-31G(d,p) level, and then analysed by GaussView 3. The calculated values are in highly consistency with the experimental ones.
Major Product, (31S,4R,6aS,9aR)-4-propyl-4,5,6,6a,9,9a hexahydrooxazolo[5,4,3-ij]quinolin-2(31H)-one,II

| Calculated
Streching Frequency/cm-1 |
Reference
Streching Frequency/cm-1 |
|---|---|
| 3032 | 3024 |
| 1836 | 2943 |
| 2865 | 2867 |
| 1825 | 1731 |
| 1051 | 1047 |
Minor Product: (()-(31S,4R,6aR,9aR)-4-propyl-4,5,6,6a,9,9a-hexahydrooxazolo[5,4,3-ij]quinolin 2(31H)-one, IIB
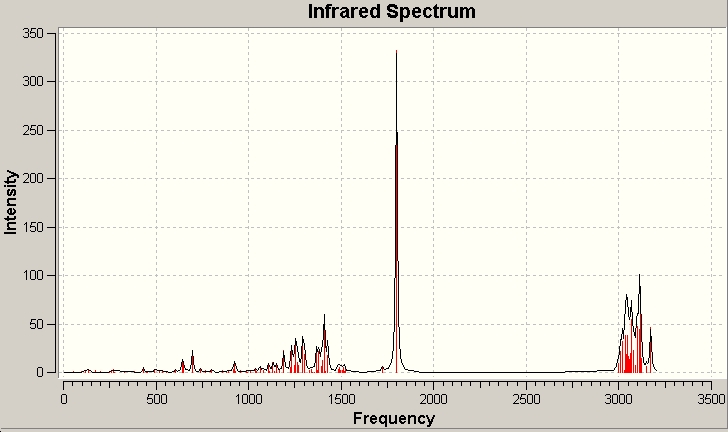
| Calculated
Streching Frequency/cm-1 |
Reference
Streching Frequency/cm-1 |
|---|---|
| 3018 | 2958 |
| 3008 | 2932 |
| 1799 | 1744 |
| 1062 | 1040 |
Conclusion
The computer modelling method based on quantum mechanics makes reasonable prediction for properties such as IR and NMR Spectrum. The result is very accurate in a large range. More compicated level of approximation usually gives better result. However, it also costs more complicated calculation. To successfully use computational modelling in real life. The most important thing is to analyse the problem and decide which level of calculation should be used and try to find out some restriction to simplify the caculations.