Rep:Mod:xxyte35134
Part 5: Regio-, Chemo-, Diastereo- and Stereoselectivity in a synthesis of Tamiflu
A.Kamimua and T. Nakano recently reported[1] a new synthesis of Oseltamivir - Tamiflu, involving an initial Diels-Alder cycloadditon between N-Boc-pyrrole and a substituted acetylene, as a preparative route for aminocyclohexenes, by elimination of the amine bridge under basic conditions. We shall analyse the synthese using modelling methods to answer the following questions of selectiviy. The reported synthesis is descirbe in scheme 1. Calculations to the DFT B3LYP/6-31G(d) level, using the GIAO NMR method where applicable.
Enantiomers of the Diels Alder adduct of N-Boc-Pyrrole and Bromoacetylene methyl ester
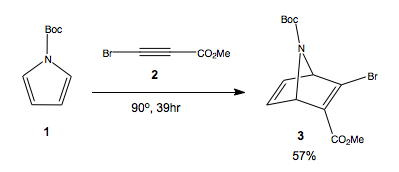
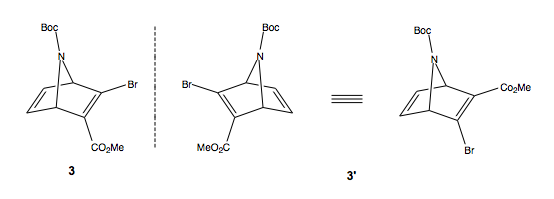
This reaction proceeds via a 4+2 cycloaddition between an electron poor, due to two electron withdrawing groups, alkyne and a formally electron rich diene. However, the pyrrole is generally unreactive in DA due to the aromaticity of the ring, making for a high energy barrier to reaction. In this paper, the authors report the use of the Boc substituent at N to make the ring more reactive as a diene. The reaction proceeds to produce a racemic mixture of the two enantiomers, 3 and 3'. The chirality is inherent due to structure of the molecule, rather than due to a chiral carbon atom. The synthesis did not resolve these enantiomers, so we have here a formally racemic syntheis of tamiflu.
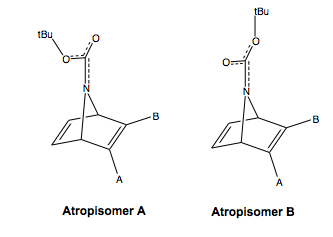
The two enantiomers also each have a pair of rotational isomers (atropisomers), shown left for one enantiomer, due to the absolute configuration of the Boc group. The N-C(Boc) bond has a high arrier to rotation, due to the amide character of the bond. The CN bond would likely rotate, but slowly compared to amine C-N bonds, because the amide character is not as high as an isolated amide, due to the delocalisation of charge also to the ester. Hence, the amide character is reduced and slow rotation would be able to take place. However, compared to the NMR time scale, this would likely be slow, so we will have to consider both atropisomers when analysing the NMR spectrum.
Why was the reaction yield here so poor, despite using N-Boc-pyrrole as the solvent, and allowing the reaction to proceed for such a long time? Perhaps the reaction was reversible? Let us compare the total energy of the starting materials to that of the product, to answer this question. The geometry of the starting materials and product (both rotational isomers) were optimised. The bromoacetylene methyl ester was found to lie in the s-cis conformation, as expected. Likewise for the Boc amide-ester. The Boc group was found to lie slightly out of plane of the C-N-C bridge, showing the reduced amide character, and again,, the two esters were s-cis. Comparing the total energies, we find that the Diels Alder adduct is 51.94Kjmol-1 more stable than the starting materials. This is a large stability gain, so this reaction will be irreversible. The the poor yield must be, at least partially due, to the relatively unreactive nature of the N-Boc-pyrrole. The two rotational isomers differ by ca.0.4Kjmol-1
Chemeo- and Stereoselectivity in the Epoxidation of Intermediate 3
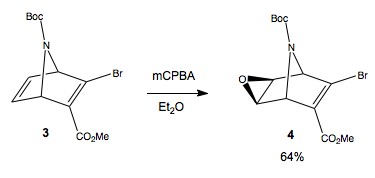
Kamimura et al. report this epoxidation of diene 3 to yield only the exo-epoxide 4. We now are going to ask, why does the reaction proceed in the observed total stereoselectivity, and why is the reaction chemoselective only for the unsubstituted alkene?
The chemoselectivity is easy to address. We have to consider the difference between the two alkenes. The substituted alkene is electron poor, due to the presence of two electron withdrawing groups. We discussed nucleophilicity of dienes with respect to chemoselective addition of electrophilic reagents (CCl2) above, and showed that the nucleophilicity of the donor pi orbital is crucial in the reactivity of the diene. In fact, this system draw many parelles to the system considreed above. The Boc group is found to tilt away from the substituted doubel bond in this molecule, much as the chloride group was. Above, we found that the exo double bond was stabilised by the sigma star donation, from C-Cl, and the endo double bond was destabilised by steric bulk, or C-Cl π* overlap. In this system, the electron withdrawing substituents on the exo doubel bond will add to the stabilising effect of the σ*, to further decrease the nucleophilicity of the alkene, relative to the endo alkene, which is unsubstituted, and destabilised by steric bulk from the tBu group. The situation is complicated by substituents, but we still ate able to identify the orbital coefficients of interest when discussing π donation by the two alkenes, which is HOMO-3. The coefficients of the unsubstituted double bond are much larger than the (almost non existent) sub double bond, so undergo total chemoselective epoxidation to the unsubstituted double bond. We can identify the stabilising C-N σ* orbital at LUMO+9, very much higher in energy than C-X sigma*, (we see the C-Br σ* at LUMO+3), and hence a weaker donor, so any stabilistaion of the exo alkene would be minimal, although it is in the correct orientation.
Now, to address the stereoselective epoxidation. Why is only this diastereoisomer formed, especially when this is the more hindered face, by the very bulky tBu group. We encountered a similar situation when looking at the stereoselective reactions of pyridinium ring systems. The addition of methyl Grignard proceeded with complete stereoselectivity for the top face, due to chelation of magnesium to carbonyl oxygen, which was tilted up, delivering the alkyl group to the top face. I wonder if something similar is happening here?
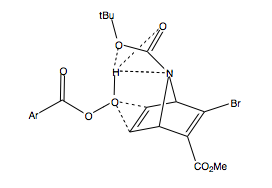
Does the mCPBA chelate or somehow associate with the Boc O (or N), to deliver the epoxide to the top face? It is known[2] that alcohols can direct epoxidation to the same face as the allylic alcohol, even if it is more hindered, by formation of a hydrogen bond between the peracid and the alchohol. A similar Hydrogen bonding could be occurring here, between the peracid H and the Boc O. This would likely be favorable, since potentially three hydrogen bonds can be set up between both oxygen atoms and the nitrogen atom of the ester-amide link. This explains the observed stereoselectivity for exo epoxidation, despite being to the bulky hindered face.
The yield of this reaction is reported as 64%, after 24hours in Et2O. The yield is low, and after reaction, unreacted starting material, and mCPBA were found. This might be due to the sterically demanding trajectory of approach of the two reactants, to ensure the OOH functionality inserts between the ring and the tBu.
Looking at the geometry of the product diasteroisomer shown, the Boc group now points up much more, as before it was far more leaning toward the unsubstituted double bond. During the epoxidation, the mCPBA may first have to force its way in, pushing the tBu up, before it can react, and then in the final product, the tBu group has rocked forward because of the increased repulsion between the epoxide O and the Boc O and N. This also helps to explain why the yield is poor, as the reaction is energetically demanding to squeeze in past the tBu.
Endo/Exo isomerism in the Hydrogenation of Epoxide 4
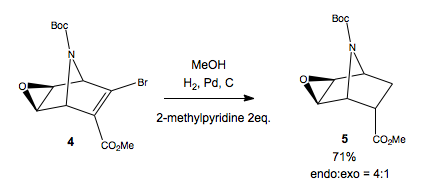
The next synthetic step involves the hydrogenation of the remaining alkene. Heterogeneous catalysis results in the syn addition of H2 across the double bond. The authors reportedly had trouble with this step, and tried a multitude of reaction conditions. Initially, they tried a simple H2/Pd/C hydrogenation. However, this resulted in the elimination of HBr, as well as hydrogenation, which then went on to cleave the N-Boc group, and the resulting azabicyclic system ring opened. The addition of amine base as a HBr scavenger resulted in the desired hydrogenation, in 80% yield, but without any stereoselectivity for the desired endo product (1:1 mix). After trying different bases, they found 2eq 2-picoline provided good 4:1 endo:exo selectivity, and no ring opening. Using this set of conditions provided the disatereoisomeric product mixture in 71% yield.
How can we understand the reported increased endo-selectivity with 2-picoline? This base, which was added as an acid scavenger, apparently somehow alters the stereoselectivity of hydrogenation. The heterogeneous hydrogenation goes by an initial complexation of one face of the alkene to the metal, much as in the DCD-model of alkene bonding to transition metal centres, but now to bulk Pd. If we look at the geometry of the starting material, we see the both faces of the alkene to be hydrogenated are somewhat hindered, by tBu and by the other half of the bicyclic ring system. However, this hindering must not be part of the rate determining step of hydrogenation, otherwise we would see a different endo:exo ratio irrespective of the base used, and also probably in a much more selective manner.
There may be two possible reasons for this:
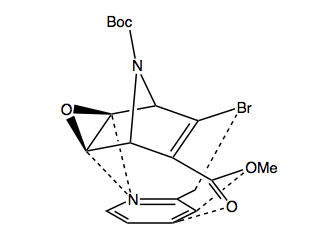
1. Transient coordination of the 2-picoline to the bottomn face of the bicyclic system.
I propose that the 2-picoline can weakly coordinate selectively to the bottomn face of the ring. By analysing the partial atomic charges of the molecule, and of 2-picoline, we find that the we can match up the charges by placing the N under the epoxide. The delta positive epoxide carbon atoms would have a Coulombic attraction for the delta negative nitrogen atom, and on the other side of the ring, the electronegative O,and Br atoms can Coulombically associate to the methyl group and the delta positive carbon atoms of the ring (relative to O and Br).
This attraction would likely be weak and transient, but it may hinder the approach of the alkene face to the metal surface, which is the rate determining step in heterogeneous hydrogenation reactions.
2. Epimerisation of the exo/endo stereocentre
Perhaps if in the the hydrogenation, some methanol was cleaved, to give the methoxy anion, a small amount of deprotonation would have occurred at the carbon alpha to the ester. This would result in the formation of a carbanion, which is planar. Then, when this reacts with more MeOH, epimerisation at that stereocentre has occurred. An equilibrium is set up, as it is catalytic in MeOH, so then slowly, more of the stable isomer will accumulate.
However, when we compare the energies of the two isomers, we find that the exo-isomer is in fact more stable by 3.52Kjmol-1, probably due placing the ester group equatorial, rather than axial. Perhaps the 2-picoline can assist to make the reaction to form endo-5 have a lower transition barrier, perhaps again by Coulombic association, so as to favor endo-5 as the kinetic product, allowing it to accumulate slightly more than exo-5 in the equlibrium mixture.
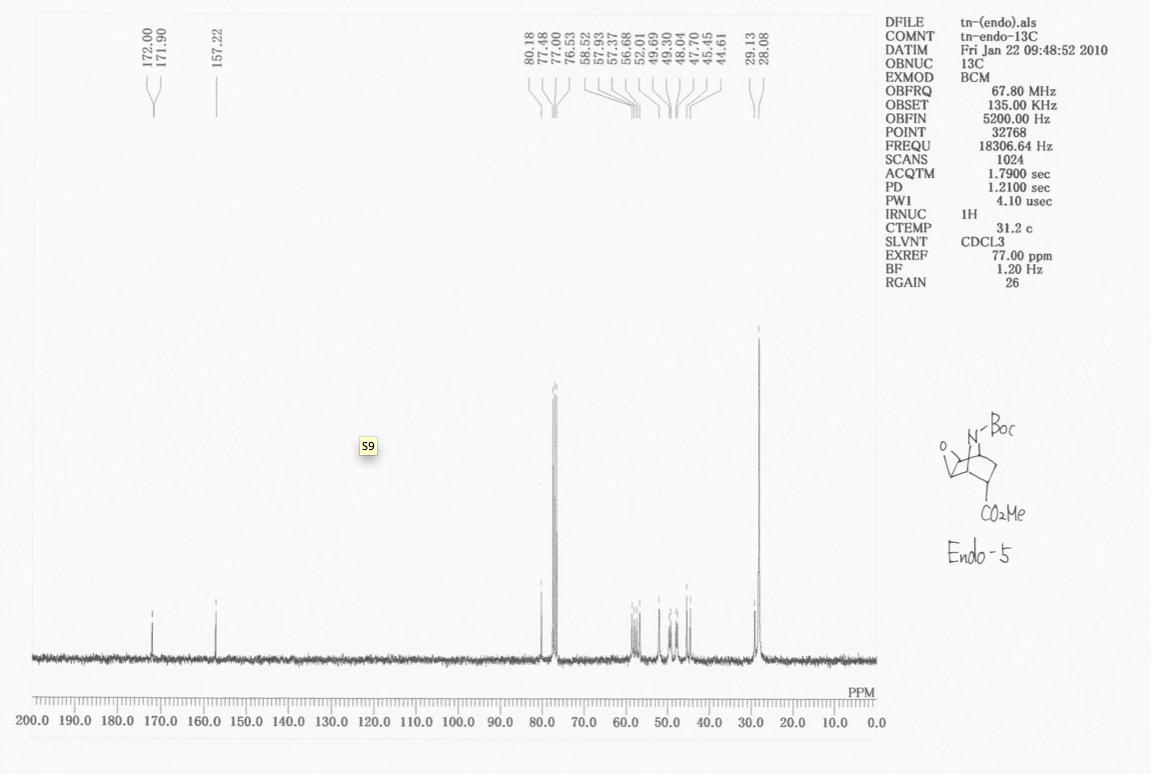
Calculation of endo and exo diastereoisomers NMR 13C shielding constants using the GIAO method
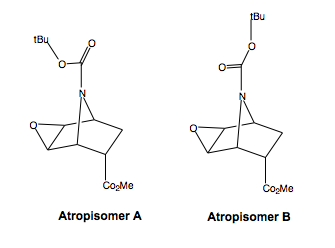
Using the GIAO method, the 13C NMR shielding constants for these two isomers were calculated. These will be compared to those described in literature. As we said above, due to the atropisomerism in the Boc group, we shall calculate the NMR shielding constants for both rotamers of the diastereiosomers.
The two rotamers chemical shifts are very similar, so the values have been averaged, since the individual peaks wouldn't have been resolved, but have combined together in a broadening effect, and the two isomers are in equal proportions, and slowly inter-converting. The shift values for the carbonyl carbon of the ester and amide-ester were corrected as described. Assuming free rotation about the O-C(tBu) bond, the shift value for the three methyl carbon atoms has been averaged.
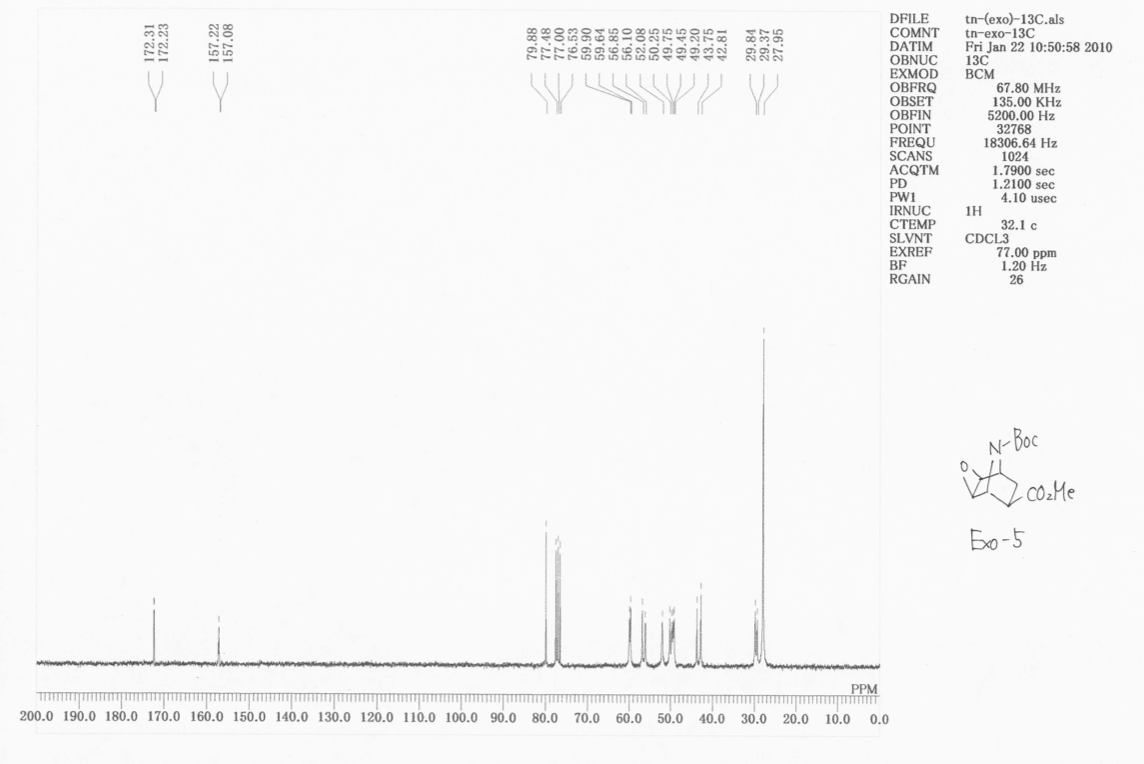
Doing the same for the exo isomer, again for the two rotamers
|
|
There is very little difference between the two sets of chemical shifts for the two diastereoisomers, except than now, we see resolution of the two highly deshielded signals into the two rotamers. Looking at the two spectra, however, we see a great change in intensity of signals of C25, the central tBu carbon. The ratio of intensities on going from endo 5 to exo 5 is 3:1. Also, more signals appear in this region. When placing the ester group up, probably due to steric bumping, the rate of rotation of the tBu is slowed. Then, we see the resolution of the three possible conformers about that bond. This is why more signals appear, and why the intensity goes from 3 (averaged environment where the tBu rotation is faster than the NMR timescale to 1 *where the rotation is slower than the NMR timescale). This is how we can spectroscopically determine the different isomers. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Literature spectra:
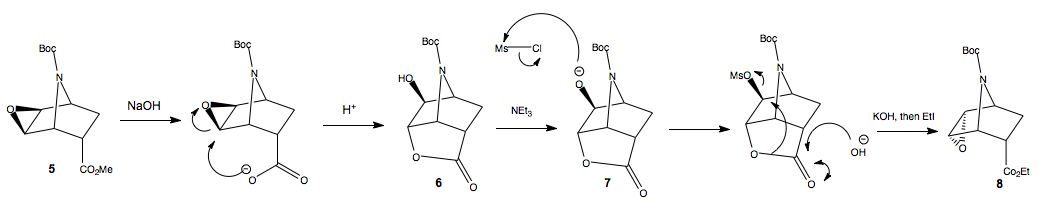
Stereospecific inversion of the epoxide exo-5 to endo-8
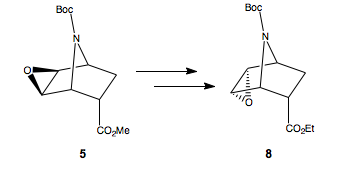
The inversion of configuration of the epoxide from intermediate endo-5 to exo-8 proceeds in a stereospecific manner, by virtue of the mechanisms involved.
Initially, base hydrolysis forms the carboxyate anion, which the opens the epoxide to form the new tricyclic lactone alcohol. Since this is endo-isomer reacting (because the diastereoisomeic mixture 4 was separated by flash chromatography), the ester is positioned to intramolecularly react with the epoxide, on the same side of the ring. Then reacting with triethylamine to deprotonate the alcohol, followed by addition of mesyl chloride to afford the good leaving group, so that when we treat with base to hydrolyse the lactone, we form the epoxide on the bottomn face as the mesyl group leaves. Then, re-esterification with EtI affords the ethyl ester.
Intramolecular elimination to ring open the bicyclic system
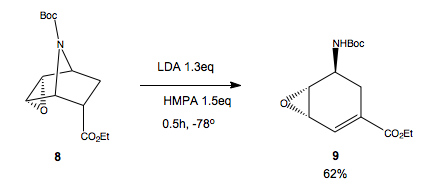
Intermediate 8 is reacted with LDA in the presence of HMPA to eliminate the NBoc and open the ring. The authors report that treatment with tBuOK resulted in no elimination, and recovery of most of the starting material. The reaction with LDA yielded 24% conversion and 38% recovery of 8. Adding HMPA into the mix made the yield of 9 62% and only 2% recovered. Why do we get this behaviour?
The pkaH of tBuOK is ca. 19. The pka of the proton alpha to the ester in 8 would be ca25. Hence, no reaction with tBuOk is seen because the base is not suffficiently strong enough to deprotonate the alpha proton. Also we see almost total recovery of the starting material because all other protons are less acidic. Next trying LDA, which has a pKaH of ca.40. Hence, this is sufficiently strong enogh to deprotonate alpha to the ester, as required for the elimination to give 9, but not to deprotonate any other protons which would have a pka of ca.45.
Now, how do we get elimination at all? looking at the geometry of the reactant, we don't have the required app arranegnt of C-H and C-NBoc to accommodate the E2 mechanism. The E1 is not likley since the NBoc is a poor leaving group. We are left with ElcB. This is promising. The deprotonation occurs alpha to an ester, hence the electron withdrawing power of it can stabilise the carbanion formed by deprotonation. Also, this explains why HMPA added to the mix gives a better yield. The Li+ cation would associate to the carbanion in a Coulombic pair, and hence reduce the nucleophilicity of the carbanion, and reduce the rate of the elimiantion of NBoc. Adding HMPA will bind to the Li+ strongly, so it doesn't associate to the carbanion, leaving it more reactive and so it eliminates the amine, to give our desired product,9.
Regiospecific ring opening of epoxide 9 with 3-pentanol
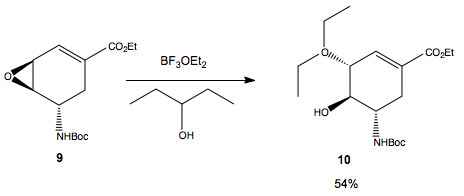
Why do we see regioselective addition of the alchohol? Looking at the geometry of the starting material, the Birgit Dunitz trajectory for approach to the carbon alpha to the amine junction involves squeezing past the tBu group of the BOC. The trajectory to the other carbon of the epoxide is unhindered, so hence we see this regioisomer as the major product.
Diastereoselectivity in the epimerisation of alcohol 10, by Oxidation-Reduction
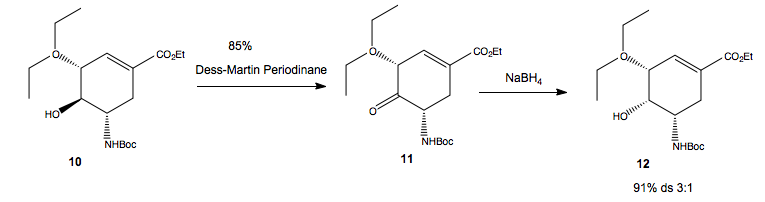
This final part of this synthesis involves epimerisation of the alcohol, by initial oxidation to a ketone, with Dess Martin Periodinate, followed by reduction to the alcohol by NaBH4. This shows some diastereoselectivity, in that a 3:1 mixture of the two diastereoisomers at alcohol are produced. Presumably, the alcohol-up isomer, which is number 10, again is recycled to get better yield of product 12, alcohol-down isomer. Why does the reduction by NaBH4 show diastereoselectivity?
If we are to compare the energies of the two diastereoisomers, then we find that placing the alcohol up, 10, is 5.59Kjmol-1 lower in energy than placing the alcohol down, 12, i.e, there is an increase in the energy of the system on epimerisation. Hence, the reduction must be a kinetically controlled process, i.e, irreversible, and the OH down epimer must have a lower energy transition state, ie,be the kinetic product, for us to see it as a major product.
If we look at the ketone, 11, we find the carbonyl group tilts down very slightly. This draws parallels to the pyridinium aniline addition earlier. We know[3] that NaBH4 prefers to approach the carbonyl group axially, and that it is anionic. The axial approach from the bottomn face is disfavored due to repulsion between the BH4- and the carbonyl group, as we saw before, so has a higher energy transition state to form than when the BH4 approaches axially from the top face, to deliver 'H-' and form the alcohol down. However, the difference in energy won't be too great, otherwise be totally diastereoseletive, as was the case for the dimerisation of cyclopentadiene.
pKa values from evans.harvard.edu/pdf/evans_pKa_table.pdf
Part 1: The Dimerisation and Hydrogenation of Cyclopentadiene
Part 2: Stereochemistry of Nucleophilic addition reactions to a pyridinium ring system
Part 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Part 4: Chemoselective Addition of Dichlorocarbene to Dienes
Part 5: Regio-, Chemo- and Stereoselectivity in a recent synthesis of Tamiflu