Rep:Mod:xxyte35133
Part 4: Chemoselective Addition of Dichlorocarbene to Dienes
Geometry and Reactivity
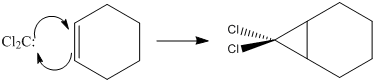
Dichlorocarbene (a singlet carbene) reacts with alkenes in a concerted (2+2 cycloaddition) manner to form dichlorocyclopropyl- derivatives. The reactant has an empty px orbital, making up its LUMO, so is electrophilic. Hence, we would expect its reactivity of addition to alkenes, and hence regioselectivity in the reaction with dienes to be determined by the nucleophilicity of the alkene donor orbital, the π HOMO. This can be adjusted by substituents, as is demonstrated here. We begin our analysis by looking a series of 1,4-cyclohexadienes with different substituents. Then we move onto look at decalin-dienes, finally placing a bridge across the two with different substituents on it. Calculations to the DFT B3LYP/6-31G(d) level.
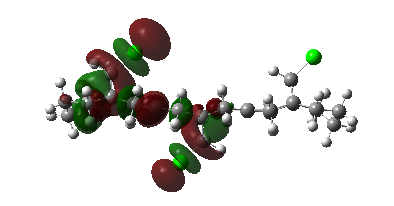
For cyclohexadiene, we see the two alkene π coefficients to be equal in size for the two double bonds, as is required since the molecule is symmetrical. We see some overlap across the ring between the two double bonds in the HOMO π orbitals - the overlap integral across the ring is just sufficient to allow for some small interaction. The ring may have contracted slightly, across the face, due to this favorable overlap.
|
1,4-Cyclohexadiene |
||
|
HOMO, z-axis |
HOMO, x-axis |
|
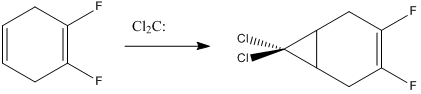
If we were to place electron withdrawing substituents onto one alkene, say fluorine, the molecule is no longer symmetrical about a plane between the two π orbitals, and hence, nor is the molecular wave function. The HOMO is now a π* type interaction between sp2C and F, which is found at a much higher energy than the alkene π interactions were in the previous system. But if we look at HOMO-1, ie, now comparable to the NBOs discussed above, we see the π orbital coefficients of the F-substituted alkene have been reduced to zero - ie, the nucleophilicity of that alkene has been 'switched off'. The unsubstituted alkene has non-zero electron density, so hence, we would expect to see total regioselectivity for dichlorocarbene(or other electrophilic reagents, like bromine or peracid) addition to the unsubstituted alkene.
|
1,2-Difluro-Cyclohexa-1,4-diene |
||||
|
HOMO, z-axis |
HOMO, x-axis |
HOMO-1, z-axis |
HOMO-1, x-axis |
|
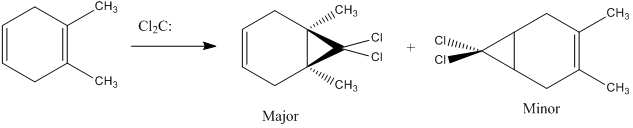
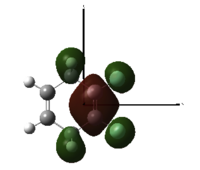
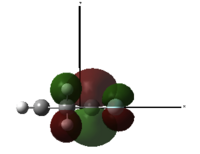
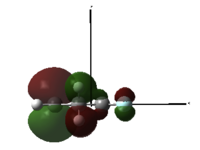
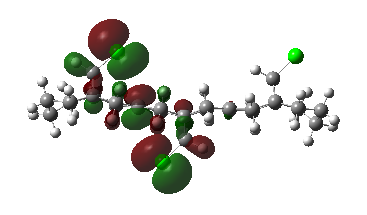
Now, placing electron pushing substituents onto one alkene - methyl groups. Looking at the HOMO, comprised again of alkene π interactions, we see the orbital coefficients of the substituted alkene are larger than the coefficient of the unsubstituted alkene. More electron density has been placed in the substituted alkene compared to the unsubstituted alkene, and as a result, we would expect dichlorocarbene to regioselectively add to the substituted alkene, since it has increased nucleophilicity. We may expect some addition to the unsubstituted alkene, since the difference in nucleophlicity is minimal, due to the weak electron pushing nature of methyl. This is provided steric bulk will not upset the trajectory of approach.
|
1,2-Dimethyl-Cyclohexa-1,4-diene |
||
|
HOMO, z-axis |
HOMO, x-axis |
|
Now, we shall move to a more complicated decalin-diene system. We shall now also use the semi-empirical PM6 calculation, and check the calculation against the more intensive DTF methods we have been using earlier. We begin by looking at the simple example of the decalin-diene. Running a MOPAC PM6 calculation, and then visualising the coefficients of MO's, we get results shown below. These make no sense. We expect the HOMO to be π orbitals, of equal size across the two double bonds, since all other NBO's will be too low in energy and the molecule is symmetrical. Likewise for the LUMO, we expect equal π* character, but again, we do not see that. The model even seems to suggest some p character in H atoms! Increasing the number of iterations did not change the result.
However, trying the PM6 calculation a second time, with the same parameters, on a different boot of the CHemBio3D program, gave a molecular surface which we expected. Perhaps the geometry this time was optimised to the point group of the molecule more precisely, allowing the molecular orbital generator a more accurate render. Then, trying the calculation a third time, this time from the PM6 optimised geometry, which produced the result that made sense, now produced another erroneous picture. Obviously there is a bug in the program. It seems though to be random, rather than related to some specific functional group or atomic parameter - as was the MM2 optimisation involving N+. In these simple cases, we can understand where the error is, but as the system grows larger, and more complex, it may not be so easy. For this reason, and because of the random nature of the appearance of this bug, I am instead going to use the more rigorous DFT B3LYP/6-31G(d) calculation. This mid-level basis will produce results that are accurate, but also not taking too long. The calculations for this size of system generally seem to take ca. 10-20 minutes of wall time.
|
1,2-Dimethyl-Cyclohexa-1,4-diene |
|||
|
Erroneous PM6 result, HOMO z-axis |
DFT B3LYP/6-31G(d) result, HOMO z-axis |
Sucessful PM6 result, HOMO z-axis |
|
|
Erroneous PM6 result, HOMO x-axis |
DFT B3LYP/6-31G(d) result, HOMO x-axis |
Sucessful PM6 result, HOMO x-axis |
|
Now, to quickly discuss the DFT results of the decalin diene optimisation. The symmetrical molecule has a symmetrical wavefunction of the HOMO, consisting of equal π character across the double bonds, as expected.
Placing electron withdrawing and electron pushing substituents onto one double bond produced the same results as above. Placing electron-withdrawing F substituents onto one double bond resulted in a HOMO-1 orbital (HOMO as before was sp2C-F π* type) with high electron density on the unsubstituted alkene and no electron density on the F-substituted alkene. Conversely, substituting in methyl groups showed the opposite trend to F-sub, increasing the coefficient of that alkene π NBO in the HOMO, compared to he unsubstituted alkene. We would expect the carbene to add to the unsubstituted alkene in the F-decline almost totally regiospecifically, with the opposite, but not as totally, regioselectivity in the methyl decalin-diene, as discussed above for cyclohexadiene-derived molecules.
We move on in our analysis to place a bridge across the joining C-C bond of the bicyclic system. The introduction of a single atom above the bridge forms a cyclopropyl-unit in the centre of the dienes - a good scaffold for the introduction of face-on substituents - to come. Looking at the localised alkene π orbitals (HOMO - degenerate) we see a symmetrical wavefunction, very much like the unbridged system above, with large coefficients across the double bonds. The other degenerate orbital, has delocalised symmetrically across the molecule, with some alkene-π cyclopropyl-π like character. The bridge - sp2C distances are all equal, at 3.08Å.
|
Bridged Decalin-diene - Degenerate ground state HOMO |
||||
|
HOMOα, z-axis |
HOMOα, x-axis |
HOMOβ, z-axis |
HOMOβ, x-axis |
|
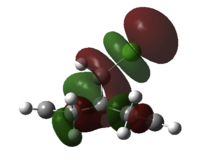
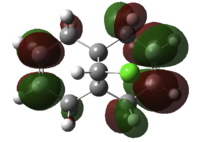
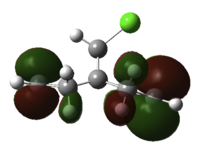
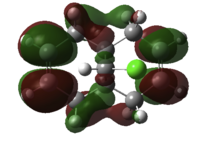
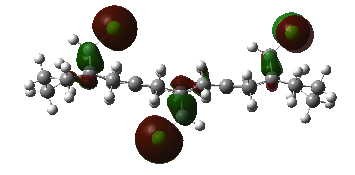
Next, we substitute a hydrogen atom on the bridge carbon for a chlorine atom. Inspecting the orbitals, we first notice the HOMO is degenerate, and made up of two types of Cl-C π* type overlap between Cl and cyclopropyl-C. Lower in energy is HOMO-1, which are the alkene pi NBO's. The LUMO is alkene π* centred, as expected, with larger orbital coefficients on the exo-double bond, than the endo- to Cl double bond. The LUMO+1 features alkene π* character, now with bigger coefficients on the endo-double bond, and opposite phase across the system. The LUMO+2 orbital is a C-Cl σ* type NBO. The endo-double bond carbon to bridge carbon distances are both measured to be 3.28Å and the exo distances are 3.03Å, a difference of 0.25Å.
|
Chloro-substituted Bridged Decalin-diene |
||||
|
HOMOa, z-axis |
HOMOa, x-axis |
HOMOb, z-axis |
HOMOb, x-axis |
|
|
HOMO-1, z-axis |
HOMO-1, x-axis |
|||
|
LUMO+2, x-axis |
LUMO+1, z-axis |
LUMO+1, x-axis |
LUMO, z-axis |
LUMO, x-axis |
How can we explain these observations? The large Cl-cyclopropyl π* type overlap, HOMO a and b, will overlap destructively with the endo-alkene pi orbital HOMO-1, hence destabilising the orbital, so raising the energy and nucleophilicity of that alkene compared to the exo alkene. This filled/filled destabilising interaction explains why the endo-C distance is longer than that for the non substituted bridged system. Also, the LUMO+2 C-Cl σ* orbital will constructively overlap with the exo pi orbital, resulting in stabilisation of the filled π - not by much because the σ* is much higher in energy. This empty/filled stabilising interaction explains why the exo-distance is shorter than that for the bridged decalin diene. Therefore, we would expect to see the carbene attack the endo-double bond regioselectively in addition, but would expect some other isomer as a minor product, since the nucelophilicity of the other alkene is not totally muted.
|
|
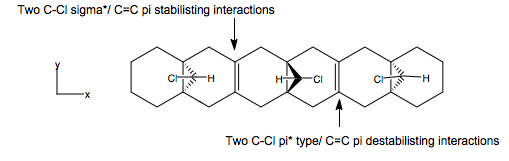
We can extend the idea of destabilising endo-overlap, and stabilising exo-overlap of the bridge Cl-C bond to this system below. Here, we have a diene , based on that above, but extended in both directions by more cyclohexane rings, to add a second and third bridge to the bottom face of the chain, the other side of the alkene. One alkene has two destabilising endo-Cl/Cπ* overlaps, and the other two stabilising exo-Cl/C σ* relationships. Here, the geometry is as above, with the endo-double bond further from the bridge head carbon than the exo-double bond (endo/exo to the central Cl). The chain is fairly linear, i.e, not curving up or down, since the pull-pull interaction on one alkene and the push-push interaction on the other will each cancel each other out, more or less.
Hexacycloheadiene system three Cl bridges |
However, a massive change is in the size of the alkene π coefficients in the HOMO. Here, due to destabilising secondary overlap between two C-Cl π* type orbitals (HOMO-1 to HOMO-3) and an alkene π orbital, and the stabilising overlap between two C-Cl σ* (LUMO+2 & LUMO+3) to alkene π orbital in the exo-double bond, the size of the endo π coefficients are much larger than that of the exo alkene, as we can see in the HOMO. Hence, the nucleophilicity of the endo-π orbital is much greater, and we would expect to see much greater regioselectivity for electrophilic attack than when we only had one set of secondary overlaps. Note that C-Cl π* type interactions make up HOMO-1 to HOMO-3, with varying orientations and coefficient of the atomic orbitals in question, all very close in energy and degenerate. Also, the two sets of C-Cl σ* orbitals are different in energy due to the different arrangements of chlorine relative to each other. LUMO+2 is made up of the two C-Cl σ* orbitals over one alkene, and the LUMO+3 the other.
|
The effect of two stabilising and two destabilising interactions on a diene |
|
|
LUMO+3 |
LUMO+2 |
|
HOMO |
HOMO-1 |
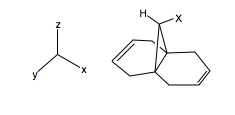
What shall now be demonstrated is the effect of the bridgehead carbon substituted on the geometry of the bicyclic system. This will allow us to infer general rules on the nucleophilicity and hence regioselectivity of addition to the diene, by electrophilic reagents. Using the general molecule shown, the substituent X was changed, and the geometry optimised. The bridge-head to endo-double bond carbon was measured, and also the same for the exo-. The angle of the substituent with respect to the carbon bridge was also measured. Unfortunately, the 6-31G(d) basis set does not include Iodine, so was not studied. The results are thus, and we compare to H:
|
Substituent X |
Endo-Double Bond Carbon Distance /Å |
Exo-Double Bond Carbon Distance /Å |
Angle /o |
|
H |
3.08 |
3.08 |
117 |
|
F |
3.21 |
3.05 |
118 |
|
CN |
3.21 |
3.05 |
122 |
|
Cl |
3.28 |
3.03 |
122 |
|
Br |
3.31 |
3.02 |
121 |
|
Me |
3.28 |
3.06 |
124 |
|
tBu |
3.39 |
3.05 |
132 |
|
SiH3 |
3.12 |
3.09 |
127 |
|
SiMe3 |
3.27 |
3.07 |
133 |
|
SiEt3 |
3.27 |
3.07 |
134 |
|
SitBu3 |
3.40 |
3.05 |
140 |
Looking first at a series of halogens. Relative to hydrogen, we see as we move down group 7, the endo-distance increases massively, and the exo-distance shrinks considerably. As we said for the Cl discussion above, the endo-distance increases due to destabilisng overlap of X-C π* type orbital with the alkene π bond. As the substituent grows in size, the orbitals become more diffuse, which would mean a less effective overlap, but also coupled with that is the increase in size of the orbital, which is then able to encroach more upon the alkene pi bond, so leading to an overall increase endo-distance. We would also expect the nucleophilicity of the alkene to increase with the geometric distortion, so down the group of halogens. The exo-distance shrinks as we move down the group. Again, there must be a balance of two effects, because we would expect the energy of the σ* C-X to increase as we go down the group, with the electronegative of the atom, and from this, expect the C-F σ* to be the best donor. However, we must also consider the overlap integral as before, which would probably increase as we go down the group, and hence see the expected trend.
We can also consider CN substituent here in the group, as the orbitals which overlap to de/stabilise the structure are the similar to those for the halogens. We see the same results as fluorine, with respect to the geometry changes, but a slightly larger angle, as the increased size of substituent pushes itself out of the system more.
Now, we consider a series of carbon based substituents. The stabilising interaction is between the exo-alkene π orbital and the C-C σ* orbital, but the σ* is now much higher in energy than for the halogens. Hence, we see much less effective shrinking with the C-Me and C-tBu substituents. Also, the destabilising interaction has been removed, since there is no π* type orbital above the ring now, but the ring does bend down considerably. However, this is a steric effect, due to the bulk of the group. This will not change the regioselectivity of attack, since the nucleophilicty is not changed by this steric effect. However, as the size of the group increases from H to Me to tBu, we would expect the selectivity for attack from the bottom face to increase, and to massively dominate when we consider tBu. The increasing size of the substituent can be seen from the increasing angle form the ring.
Comparing CH3 to Si3, we see the distances endo are a lot smaller for SiH3, and we can understand that by looking at the angle from the ring - increased, so the steric bulk has been removed somewhat. Looking at the exo-distaces, we can see that the C-X σ* donating power of the Si is weaker (higher energy) than the carbon.
Comparing a series of SiR3, as the size of the R group increases, the change in geometry of the endo-ring increases, with sterics as explained. Likewise, the angle out of the ring increases too. As the size of R increases, the exo-distance shrinks, by a small amount. The energy of the donor C-Si σ* must be being lowered, or the overlap between the Si-C σ* and alkene pi increases. This could be the case, because the angle out of the ring increases with size of R, and this may lead to the overlap integral of the secondary overlap increasing too, hence, a more stabilising interaction.
Anaylsis of the vibrational modes of Bridged decalin dienes
We are going to calculate the influence of the Cl-C bond on the vibrational frequency of the Chloro-substituted bridged decalin diene, 12, and its exo-dihydro derivitive. However, for simplicity, we shall begin with a vibrational analysis of the unsubstituted bridged diene.

This diene is of the of the C2v point group. Hence, we can only generate vibrational modes which are allowed under the point group symmetry. Therefore, we will only see vibrations which are mirrored across the planes of symmetry, either symmetric or antisymmetric. Also when analysing the specturm, we have to take into account the selection rule for vibrational modes: A vibraional mode is only infra red active if it results in a change of diploe moment on excitation.
Let us now look at the calculated infra red spectrum for the diene.
At 3230.1cm-1, we see a vibration resulting from antisymmetric stretching of the bridge C-H bonds about the sigma v (yz) plane. The transition is weakly allowed, because although the dipole moment does change on exciattion, because the strecth is antisymmetric, because we see hydrohen atoms shifting, they have little influence on the dipole moment. Its 'sister' vibration, where the CH bonds strecth in a symmetric fashion is formally disallowed because of the lack of dipole change on excitation. However, we see it weakly at about the same intensity as the AS stretch, at a lower energy of 3133.6cm-1. The carbon atom recoils slightly on vibration, so this may be why we see the weak transition again, becasue the diopole changes a little.
At 3173.6cm-1, we see the AS stretch of the alkene CH bonds about the sigma v(yz) plane. This is strongly allowed, intensity 103.6, since the dipole moment has shifted on vibration. Just as this is strongly allowed, the sister S vibration at 3174.3cm-1 is strongly forbidden because the dipole moment doesnt change, and the mode is vibrationally inactive, intensity 0.3
At 3021.4 and 3020.6, we see two equally intense signals resulting from an antiymmetrical and its sister symmetric stretching mode of the sp3 C-H bonds about the sigma V (xz) plane, and antisymmetric about the plane of the ring. For these bonds, because they are out of the plane of the ring, the dpole moment changes on excitation, by the same amounty for each mode, so we see two equally allowed transitions.
At 2994.7 and 2993.3, we see two more intense signals resulting again from an antiymmetrical and its sister symmetric stretching mode of the sp3 C-H bonds about the sigma V (xz) plane, but now also symmetric across the plane of the ring. The AS strecth is much more intense, because the dipole moment changes much more on transiton.
The alkene C=C strecthing vibrations about the sigma v (yz) plane are formally optically inactive because neither change the dipole moment. These are calculated to be at 1742.7cm-1(S) and 1736.8cm-1(AS), and the intensities are 0.0 and 7.2. The AS mode is weakly allowed (though formally forbidden) because the rings recoil slightly and the dipole moment will change a little.
The lower energy C-C strecthing and CH, CC bending modes are moslty forbidden or only weakly transmitting due to no change in dipole moment. Only one low energy mode has any large intensity, at 678.7cm-1, corresponding to a symmetrical CH bending mode across both mirror planes. All H atoms siumultaneouslybend up or down, hence the dipoole moment will chaneg and the transition is allowed. The signal is of low intensity because the ythe low energy of the mode.

Now we chall substitute a Cl atom for a bridgeing C hydrogen atom, as we did before, and see how the vibrations change. On substituetion, the point group changes to Cs. Now the only symmetry element (other than E) is a mirror plane bisecting the two rings. Hence, the virbational modes now can only be about this plane, unlike above, where they could be about this plane, or another bisecting the two rings in the middle. Again, we have the condition that for a mode to beoptically activ, the dipole moment must change. With the introductionn of chlorine the dipole moment of the molecule will have increased, so now it is more sensitive to changes in moment, so more formally disallowed modes will be weakly active, as the slight chnge in dipole moment will be felt more than for the CH2 bridge.
Comparing the speta, we see that apart from making alot more very low intensity signals appear, as explained abouve, the apearance changes little, becaue most of the stronly allowed transitions about were about the XXX plane, whioch is still present. We do now see the previouslt inactive sister modes, because the unsymmetrical placement of Cl has meant the dipole will now change. The energy of the C-C and CH strecthing modes are comparable to those above.
At 1757.4 and 1737.1cm-1, we see the two alkene C=C stretch. Only one now at a time, as before both did, due to the mirror plane between the two. This is now removed by addition of Cl, as explained. These are both disallowed, as they are symmetric about the plane, and hence the dipole moment will not change, so we see intensities of 3.9 and 4.2 respectively. The higher energy mode is the double bond endo to Cl, which supports our analysis of the destabilising interations above.
We do see the appearance of a new signal at 771.0cm-1. This is from the C-Cl strecth. A low energy mode due to the weak bond, because a pi* type filled bond is between them, reducing the bond order, as we saw above. This is strongly allowed, since the dipole changes massively as the electronegative atoms moves.

Now to compare to the exo-dihyrdo-derivitive. The effect of hydrogenation of the exo bond is to pucker the ring, hence removing tyhe only remaining syttetry emeenelt of the molecule, the morror plane. Now, the moelcule has no symmetry (excpet E), and is in poijnt group C1. Now, any mode is allowed, by symmetry, so we would expect the spectrum to become alot more complicated, with more signals. The dipole selection rule still stands, so that vibrations about the dipole moment will be optically inactive. This will be fewer though, because the restriction to allowing the ring to twist is now removed, and hence the dipole can be moves slightly easier, by this ring twist of the cyclohexane ring, so that we expect to see many more weeakly allowed transitions. These ring tristing modes appear around 1500cm-1, and were not seen in either of the above spectra.
The C-Cl strecthing mode is strongly allowed at 775cm-1, very similar to that before. The energy is slightly higher, duie to the loss of stabilising interaction with the exo pi bond. The remainign C=C strecthing frequency is seen at 1758.1cm-1. This compares to 1757.4cm-1, almost exactly the same as before. This is because the destabilising C-Cl pi*/C=C pi interaction remains, and raised the energy of the orbital relative to that of the unsubstituted bridged system.
Part 1: The Dimerisation and Hydrogenation of Cyclopentadiene
Part 2: Stereochemistry of Nucleophilic addition reactions to a pyridinium ring system
Part 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Part 4: Chemoselective Addition of Dichlorocarbene to Dienes
Part 5: Regio-, Chemo- and Stereoselectivity in a recent synthesis of Tamiflu