Rep:Mod:xxyte35131
Part 2: Stereochemistry of Nucleophilic Addition Reactions to a Pyridinium ring
The Stereospecific Reaction of a Tricyclic Prolinol Derivitive
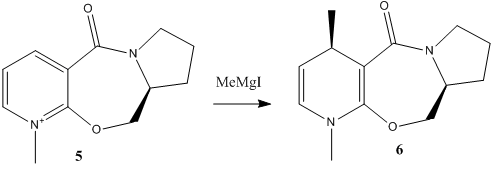
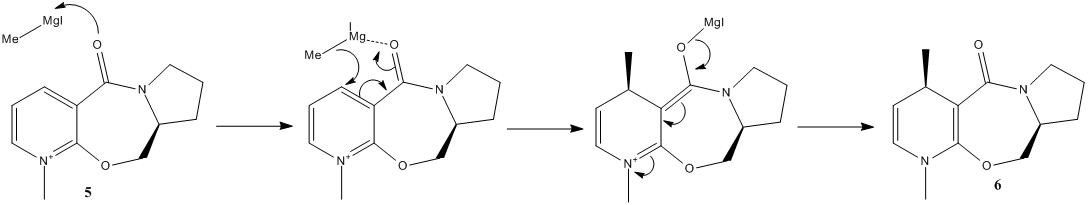
In the following stereospecific reaction, it is observed that the absolute stereochemistry of the product is that shown in product 6; placing the methyl group in the 4-position up, as drawn. Why is this methyl group placed upon this face of the ring system? Using MM2, and then a more rigorous DFT method, we hope to find that out.

Using MM2 (with ChemBio3D 12.0), the geometry of the reactant, 5, was optimised . With the constraint that the 7-membered ring be up at the tertiary carbon atom shown, several energy minima were located. Two different minima were dependent upon the geometry of the cyclopentane ring, and which atom puckers up. The conformer where atom C1 as shown puckers down, with respect to the rest of the ring, is found to be 6.01kJmol-1 lower in energy than the conformer with the atom puckered up with respect to the rest of the ring. Of this energy difference, 4.46kJmol-1 is due to torsional strain. The puckering up causes one bond to move to a position to cause a more eclipsing interaction between two neighboring hydrogen atoms, which is responsible for this increase in energy.
In the lower energy of the two ring-puckering conformers, looking at the 7-membered ring, we see it is quite conformational fixed by virtue of the nitrogen atom and the carbonyl group having rigidly fixed geometries. The amide group is necessarily planar, and the ether oxygen atom infers a kink in the ring. Using MM2, the mimima placed the carbonyl group coplanar to the aromatic system.
Schultz and Flood[1] report that the mechanism of the reaction first involves the initial coordination of the Magnesium atom of the Grignard to the carbonyl oxygen, to then deliver the methyl group to the top face of the pyridinium ring. However, if the carbonyl is planar to the aromatic ring, as the MM2 field predicts, then we would expect the product to be a racemate. However, ChemBio3D reportedly has a bug in the MM2 force-field, regarding cationic nitrogen, giving false minima. This would seem to be the case here. So, to test this, the geometry optimisation was repeated, but this time, using a more rigorous calculation. A DFT B3LYP calculation, with a 6-31G(d) basis set produced the following result. Shown here is the pyridinium reactant 5.
Prolinol-Derivitive Pyridinium system geometry |
The amide is still planar, and the ether oxygen again makes the ring kink, but now we see the carbonyl group twisted out of the plane of the aromatic ring, toward the top face as shown. Also, the C1 atom as shown is found to pucker down, which from our analysis of the MM2 geometry, was found to be the lower energy conformed, compared to C1 up. In this conformation, when the magnesium coordinates, the methyl is delivered to the top face, as reported. The mechanism goes by that shown in scheme X. Initial coordination of oxygen to magnesium, followed by a 6-membered transition state to give a magnesium enolate, which falls apart to give the diene. Presumable the diene is stable compared to the aromatic system (and we don't see loss of H+ in a Meisenheimer fashion) because one alkene is stabilised by conjugation to the amide (weak because of the non-ideal overlap), but more importantly, the compound is now neutral.
Atropisomerism in a Tetracyclic Pyridinium System, and the Reaction with Aniline
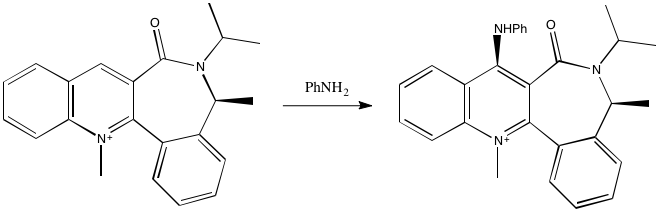
Now, looking at the addition of aniline to a pyridinium system. Using a DFT B3LYP/6-31G(d) calculation, because the MM2 previously proved to be flawed when handling N+, the geometry of the molecule 7 was optimised - shown below. Compared to the previous pyridinium system, we now see the opposite geometry of the carbonyl group - twisted down, with respect to the pyridinium ring as drawn. This is a necessary condition if the methyl group alpha to the amide is to point up, since the amide is necessarily planar. This methyl group, highlighted also means the phenyl- substituent is pointing down, if the tertiary carbon atom is to be tetrahedral. These factors mean the 7-membered ring is conformationally fixed. The isopropyl-methyl termini twist to adopt staggered conformations.
The methyl group pointing up means the phenyl- group goes down. To reverse this, the phenyl- group would have to become planar with the pyridinium system, the 7 membered ring would have to also become planar, because the amide link would have to stay planar, and this would likely be a very high barrier due the the extra bending strain in the ring, and the steric bumping between phenyl- and N-methyl. This barrier would be high. Hence, this compound is likely optically active, by virtue of the high rotational barrier. This compound, and its mirror image, formed with the methyl down and the phenyl- ring up, are atropisomers.
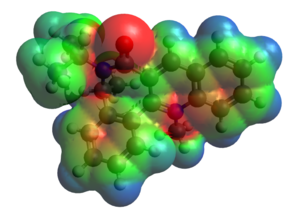
Why does the aniline attack from the top face? As the carbonyl group twists gown, the steric bulk placed around the 4-position of the pyridinium ring will increase, so this could mean the aniline favors the top face. Also, though, we should consider Coulombic repulsion between the lone pair of the nitrogen and the lone pairs of the oxygen of the carbonyl, with would operate if the nitrogen nucleophile was to approach from the bottom face. This is probably the dominant effect, because the carbonyl group does not wholly block the site of attack. The combination of these two factors explain the observed stereoselectivity. Shown left is the electrostatic potential surface of reactant 7 at then Van der Waals radius, from the bottom face, showing the high negative potential at oxygen.
Proline-Derivitive Pyridinium system geometry |
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
Part 1: The Dimerisation and Hydrogenation of Cyclopentadiene
Part 2: Stereochemistry of Nucleophilic addition reactions to a pyridinium ring system
Part 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Part 4: Chemoselective Addition of Dichlorocarbene to Dienes
Part 5: Regio-, Chemo- and Stereoselectivity in a recent synthesis of Tamiflu