Rep:Mod:xxyte35130
Part 1: The Dimerisation of Cyclopentadiene and it's Hydrogenation
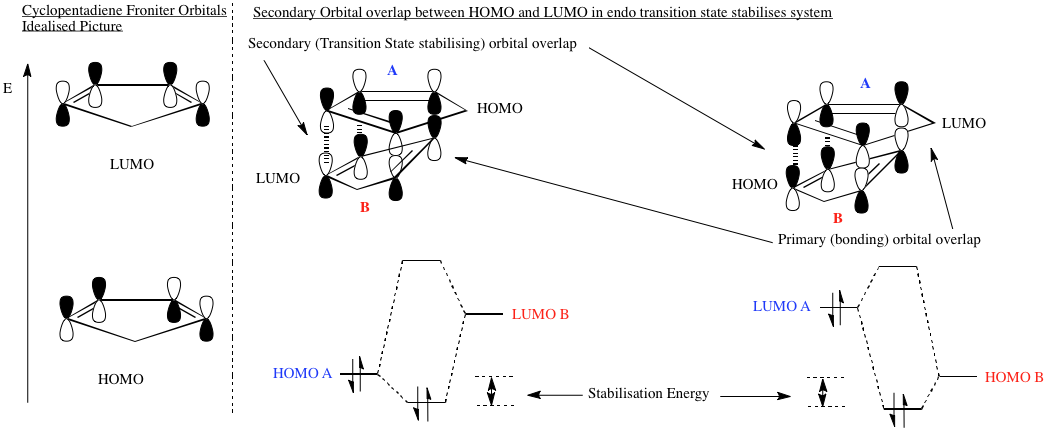
Stereospecificity in the Cycloaddition of Cyclopentadiene

At room temperature, cyclopentadiene exists as a dimer, via a π4s + π2s cycloaddition. The symmetry allowed products of this are shown. However, only one of these isomers, the endo-isomer, 1 is found in a sample. We now attempt to find out why.
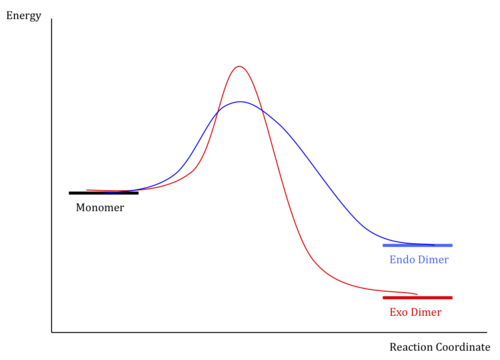
Using an Molecular mechanics optimisation with an MM2 force-field, the relative energies of exo- and endo-isomers of the dicyclopentadiene, were calculated. The endo-dimer, 1, is found to be 8.92Kjmol-1 higher in energy than the exo dimer. Given that this is the product formed, this reaction must be kinetically controlled and irreversible, to produce the less stable isomer. We can draw an qualitative reaction energy profile for this reaction.
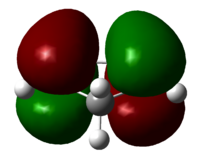
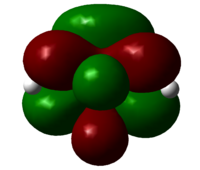
To consider why the endo-dimer is higher in energy, if we look at it as a space-filling model, we see steric bumping across the molecule, between non-bonded carbon atoms shown in blue, hence increasing the energy. The distance's between non-bonded carbon atoms across the system is shorter than the ideal Van der Waals non-bonded C...C distance of 3.1Å[1], hence this interaction is repulsive, raising the energy if the system, relative the the exo-dimer, which does not have this clash, because the atoms in question, highlighted, are removed from each other.
Endo-Isomer |
Exo-Isomer |
Now, to consider why the transition state for the endo-isomer is more stable than that for the exo-isomer. Woodward and Hoffman proposed[2] an explanation for the observed preferential formation of the endo-isomer, based upon secondary orbital overlap in the transition state. When the two rings approach face on, favorable HOMO/LUMO mixing occurs at the other end of the system to that which is bonding. In the exo-transition state, there is no secondary orbital overlap, so no stabilisation.
The frontier orbitals for cyclopentadiene shown above are the idealized case. If we calculate orbital coefficients ab initio, we see some σCH contribution to the LUMO. However, the CH2 unit is not face to face with the other ring in the transition state, so we would not expect any (considerable) orbital overlap. Calculated using B3LYP theory with a 6-31G(d) basis set.
We should finally say that this cycloaddition is reversible. Heating the endo-dicyclopentadiene to 150o[3], then collecting in a cold trap, will reverse the reaction - this is cracking, an essential first step for any synthesis involving cyclopentadiene, for example in the synthesis of ferrocene. Kept sufficiently cold, the cracked cyclopentadiene will stay as a monomer - there is not sufficient energy to reach the endo-transition state . Increasing the temperature to room temperature, there is just enough energy to form the endo-dimer, so the sample will slowly dimerise to give this product, as discussed. It is found that by heating a sample of freshly cracked cyclopentadiene, some exo-isomer is formed[2], but again mostly endo-. This reaction shows that the exo-dimerisation is still thermally, and symmetry allowed, but due to the high transition barrier, higher than endo-, the higher temperature is required, and this also raises the rate of formation of endo- too, so we still see mostly endo-, but now also a small amount of exo-.
Chemoselectivity in the Hydrogenation of Dicyclopentadiene
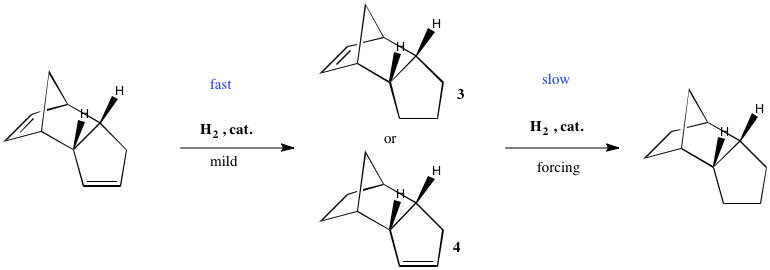
It is observed that only prolonged hydrogenation of endo-dicyclopentadiene under forcing conditions will hydrogenate both double bonds. However, if a sample of endo-dicyclopentadiene is hydrogenated for a short time, only one of the two double bonds is hydrogenated. But which one? We now attempt to find that out.
During the following discussion, I do use some energy comparisons between the cycloaddition dimer and isomers of the dihydro-product. Therefore, we should take the results with a pinch of salt, as these are not isomers, although we would expect it to be valid, as only two hydrogen atoms have been added to the system. Even so, the result validity will be tested at the end by considering between the two dihydro-isomers directly, and we shall see if we get the same result.
Using MM2, both possible structures of endo-dihydrodicyclopentadiene, 3 and 4, were optimised, and the energy difference between the two calculated. The isomer where the double bond alpha to the bridge is hydrogenated, 4, is found to be 18.98Kjmol-1 more stable than the isomer where the cyclopentene ring is hydrogenated. We can explain this by comparing the bond angles of the double bonds being hydrogenated, to those in the cycloaddition dimer.
In the cycloaddition dimer, 1, the bond angles indicated are measured to both be 107.7o. Given that this is a double bond, with sp2 hybridised carbon, the bond angles observed are somewhat smaller than the optimal 120o, hence the ring is strained. Also the bridging sp3 carbon has an angle of 93.6o, again, considerable less than the optimal tetrahedral 109o. This again introduces strain into the system.
Dimer |
Isomer 3 |
Isomer 4 |
On hydrogenation of this double bond, the two sp2 carbonm atoms rehydridise to sp3, and the angles change to 102.5o and 103.3o. These are not ideally tetrahedral, but closer than the sp2 carbons to their optimal positions. Looking at the bridging carbon, we see the bond angle is 92.6o, about the same as before. So overall, the strain in the system is reduced upon hydrogenation of the double bond in the bridged system,to give isomer 4.
Considering the bending component of the total energy, on hydrogenation to isomer 4, this decreases by 26.57Kjmol-1, showing a large reduction in strain, which is in agreement of our analysis of bond angles. However, we also see an increase in torsional energy of the system, by 12.51Kjmol-1. This is because two hydrogen atoms have been introduced into the system, so the number of H-H overlaps on adjacent C atoms, whether eclipsed or staggered, has increased. The number of eclipsing (whether totally or not) interactions has increases, so the torsional energy has increased. The overall energy difference between the dimer and the dihydro-product, 4, is therefore due to a balance between bending and torsional strains, and the bending term wins out - the stability gained by reducing strain in the system outweighs the stability decease from more eclipsing interactions.
Doing the same analysis for isomer 3, the angles in the double bond of the cyclopentane ring change from ~112.3o to 103.3o on rehybridisation. Again, the bond angles are not ideal before hydrogenation, so hydrogenation would have relieved some strain, but the deviation from ideality is less severe in this ring, so the stability gain on hydrogenation is less. The bending (strain due to deviation from ideal bond angles) component to the energy decreases by 4.26Kjmol-1 on hydrogenation, showing the reduction in strain. Also to consider is the torsional strain. Again, this increases due to the increase in number of hydrogen atoms in the system, and the increased number of eclipsing interactions. On hydrogenation, the torsional strain increases by 5.46Kjmol-1.
For isomer 4, we see a large reduction in strain of the system on hydrogenation. For isomer 3, a small reduction in ring strain is observed. For both systems, due to an increase in eclipsing interactions from the new H atoms, the torsional strain increases. However, for isomer 4, the increase in stability from ring strain relief outweighs this increase in torsional energy. This is not the case in isomer 3. Therefore, we would expect isomer 4 to be formed quickly, under mild conditions. Isomer 4 is both the kinetic and thermodynamic product. The forcing conditions are required to hydrogenate the second double bond because the system increases in energy overall, so is not a favorable process.
Interestingly, for the dihydro-isomer 3, with the 5-membered ring double bond hydrogenated, there were found to be two closely related, in terms of geometry and energy, mimina. One with the C5 apex up, and one with the C5 apex down. The apex up conformer is 1.03Kjmol-1 more stable than the apex down isomer. This up conformer is the one discussed above. When the apex, marked purple, is down (less stable) the dihedral angles down the C-C bonds of the ring are 43.8o and 43.4o, i.e between gauche and periplanar. When the apex puckers up (more stable) the torsional strain is reduced somewhat, as overall, the number of staggered relationships increased. There are some interactions in which the eclipsing increases, but overall, compared to the apex down conformer, the up conformer is more stable as the conformation is more staggered. This can be demonstrated by looking at the torsional component of the energy, which decreases by 5.23Kjmol-1 on puckering up. The fact that this is different to the overall energy difference shows that on puckering up, some stability is lost; looking at the energy components, the bending component increases by 3.54Kjmol-1 on puckering up. So overall, the conformational preference for the cyclopentane ring is determined by a balance between torsional (eclipsing vs staggered) interactions, and bending (ideal angle) interactions. The torsional strain relief is greater than the bending energy gain on puckering the ring up, so that is the most stable conformation.
More stable, puckered up conformer |
Less stable, puckered down conformation |
The other dihydro-isomer 4 was only found to have one stable minima, because the cyclopentene ring is held planar by the double bond, and the bridged ring ring is also kept conformational fixed by the bridging carbon atom.
This above discussion has used energy comparisons across different molecules - the diene and the dihydro-derivative, but if we look directly at the two possible dihydro products and compare between them only, we reach the same conclusions. We see the ring strain (bending) energy component is 22.3Kjmol-1 less comparing isomer 4 to 3. Similarly, the total is 18.99Kjmol-1 less comparing isomer 4 to isomer 3. Therefore, because isomer 4 is more stable, it is the thermodynamic product. Because the ring strain is less in isomer 4, a greater gain in stability must result from hydrogenation since they both come from the same reactant, so we expect it is also the kinetic product. Isomer 4 is formed by mild hydrogenation.
- ↑ H.Rzepa, http://www.ch.ic.ac.uk/local/organic/conf/
- ↑ 2.0 2.1 2.2 T.L Gilchrist and R.C Storr, Organic Reactions and Orbital Symmetry, 1972, p.99 Cite error: Invalid
<ref>tag; name "two" defined multiple times with different content - ↑ Imperial College London, Year Two Spring Term Synthesis Lab Manual, 2010
Part 1: The Dimerisation and Hydrogenation of Cyclopentadiene
Part 2: Stereochemistry of Nucleophilic addition reactions to a pyridinium ring system
Part 3: Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Part 4: Chemoselective Addition of Dichlorocarbene to Dienes
Part 5: Regio-, Chemo- and Stereoselectivity in a recent synthesis of Tamiflu