Rep:Mod:xm1213
Computational Chemistry:Transition States
Introduction
During this module, transition states of a variety of pericyclic reactions at different levels will be located and characterised with GaussView. The mechanism and selectivity will be explained with vibrations and energies of the transition state.
Exercise 1: Reaction of Butadiene with Ethylene
Molecular Orbitals of cis-Butadiene and ethene
Molecules of cis-Butadiene and ethene were optimised to a minimum at PM6 level and Molecular Orbitals were generated.
| File Type = .chk |
| Calculation Type = FOPT |
| Calculation Method = RPM6 |
| Basis Set = ZDO |
| Charge = 0 |
| Spin = Singlet |
| Total Energy = 0.04691424 a.u. |
| RMS Gradient Norm = 0.00003580 a.u. |
| Dipole Moment = 0.0732 Debye |
| File Type = .chk |
| Calculation Type = FOPT |
| Calculation Method = RPM6 |
| Basis Set = ZDO |
| Charge = 0 |
| Spin = Singlet |
| Total Energy = 0.02511137 a.u. |
| RMS Gradient Norm = 0.00002852 a.u. |
| Dipole Moment = 0.0000 Debye |
Straight optimise a guess TS
The planes of the molecules were kept parallel and distance between the carbon pairs 1-14 and 8-11 were set around 2.2Å. The TS optimisation was calculated at PM6 level with a force constant.
C-C bonds length and hybirdisation
| Bond of Molecule | Length in reactant | Length in TS | Length in product | sp2 lit.[1] | sp3 lit.[2] |
| cis-Butadiene C=C in TS | 1.33 | 1.38 | 1.50 | 1.33 | 1.54 |
| cis-Butadiene C-C in TS | 1.50 | 1.41 | 1.33 | 1.33 | 1.54 |
| ethene C=C in TS | 1.33 | 1.38 | 1.54 | 1.33 | 1.54 |
| partially formed bond in TS | n/a | 2.11 | 1.54 | 1.33 | 1.54 |
The partially formed bond is longer than either bond of hybirdisation and the bond length is larger than the Van der Waals radius 1.7Ǎ of Carbon atom[3], indicates the the bond is forming. The length of double bond in both cis-butadiene and ethene is longer than literature value, and the length of the single bond of cis-butadiene is smaller. Both indicate that the bonds are on its midway to new hybirdisations. The optimised product cyclohexene has similar value to the literature, indicating that the reaction is completed.
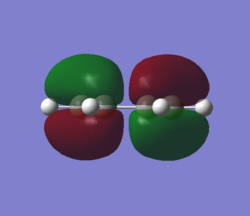
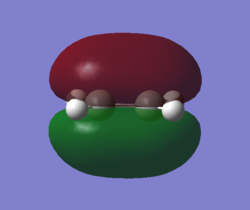
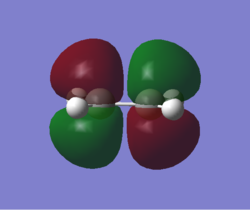
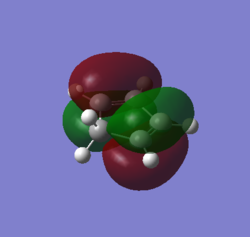
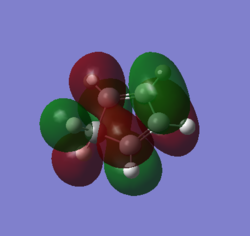
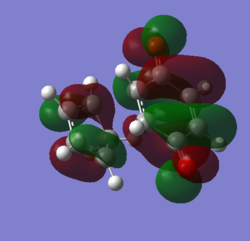
MOs and vibrations of the transition state
| Molecule | TS cyclohexene | TS cyclohexene |
| MO | HOMO | LUMO |

|

| |
| Symmetry | Symmetric | Symmetric |
| Energy a.u. | -0.32533 | 0.01732 |
The HOMO of the TS showed the symmetric π-orbitals of the butadiene interacting with the symmetric π orbital of ethene. The symmetries of the orbitals indicate the reaction is allowed. The LUMO of the TS showed the symmetric π*-orbitals of the butadiene interacting with the symmetric π orbital of ethene. Also the symmetries of the orbitals indicate the reaction is allowed. Since there were more antibonding component in the LUMO and more bonding component in HOMO, HOMO is more stablised and lower in energy.
The characteristic vibration which with an imaginary frequency of -948.56cm-1, shows the a synchronous formation of the bonds. However the lowest positive frequency demonstrate asynchronous vibration.
Nf710 (talk) 16:50, 8 December 2016 (UTC) This section was done ok you missed out a few key things like an MO diagram
Exercise 2: Reaction of Benzoquinone with Cyclopentadiene
Reactants optimisation
Molecules of the reactant Benzoquinone and Cyclopentadiene were optimised at PM6 level and then refined with B3LYP/6-31G(d).
| File Type = .chk |
| Calculation Type = FOPT |
| Calculation Method = RB3LYP |
| Basis Set = 6-31G(D) |
| Charge = 0 |
| Spin = Singlet |
| Total Energy = -194.10106256 a.u. |
| RMS Gradient Norm = 0.00006295 a.u. |
| Dipole Moment = 0.4371 Debye |
| File Type = .chk |
| Calculation Type = FOPT |
| Calculation Method = RB3LYP |
| Basis Set = 6-31G(D) |
| Charge = 0 |
| Spin = Singlet |
| Total Energy = -381.45168107 a.u. |
| RMS Gradient Norm = 0.00013109 a.u. |
| Dipole Moment = 0.0001 Debye |
Pre-Guess Transition States and freeze reacting pair
Frozen Coordinate Method was used for this exercise and the molecules of reactant were placed in either the endo or exo orientation. The reacting carbon pairs were frozen with redundant and the geometries were optimised to minimum at PM6 level. After that, both geometries were optimised as transition state with force constant at PM6 level and refined at B3LYP/6-31G(d) level
Optimised transition states
| File Type = .chk |
| Calculation Type = FREQ |
| Calculation Method = RB3LYP |
| Basis Set = 6-31G(D) |
| Charge = 0 |
| Spin = Singlet |
| Total Energy = -575.52653489 a.u. |
| RMS Gradient Norm = 0.00000373 a.u. |
| Imaginary Freq = -440.90 cm-1 |
| Dipole Moment = 2.7679 Debye |
| File Type = .chk |
| Calculation Type = FREQ |
| Calculation Method = RB3LYP |
| Basis Set = 6-31G(D) |
| Charge = 0 |
| Spin = Singlet |
| Total Energy = -575.52914752 a.u. |
| RMS Gradient Norm = 0.00000433 a.u. |
| Imaginary Freq = -439.80 cm-1 |
| Dipole Moment = 3.1960 Debye |
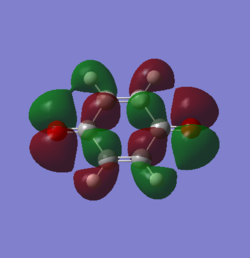
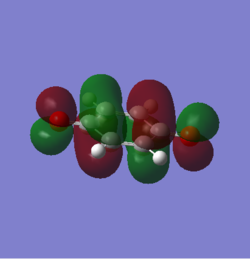
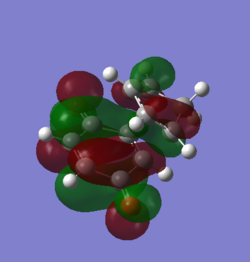
MOs of the transition states
From the graphs, endo MOs are found to have a better overlap then exo ones. The endo transition state is expected to be more stablised than exo.
IRC analysis
| orientation | endo | exo |
| graph | 
|
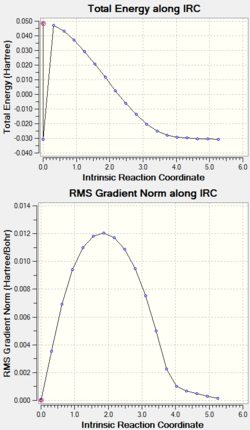
|
| IRC log file | endo TS IRC PM6 N200.log | exo TS IRC PM6 N200.log |
The endo IRC graph shows that after 200 points, the geometry of minimum energy has been found, the RMS gradient gets zero and the reaction has reached the minimum. The exo IRC was ran for three times and all ended abnormally with error link 2070, and from the graph the minimum has not been found.
Energies of the TSs and selectivity
The thermodynamics is compared by the Gibbs free energy, which is labelled "Sum of electronic and thermal Free Energies" in the log file.
endo TS b3lypd.log exo TS b3lypd.log
| Orientation | Gibb's Free Energy(a.u.) |
| Endo TS(Kinetic) | -575.383855 |
| Exo TS(Thermodynamic) | -575.381307 |
| Difference in Energies(a.u.) | -0.002548 |
-0.002548 a.u.=-6.68977451 kJ/mol
After comparing transition states at B3LYP/6-31G(d) level, endo TS is found to be more stable by 6.68977451 kJ/mol. And exo product is known to be more stable and lower in energy for being less sterically hindered, however despite being more thermodynamically stable, the Diels-Alder reaction is controlled by the kinetics which means the low energy transition state is prefered and endo product is formed rather than exo product.
Nf710 (talk) 17:06, 8 December 2016 (UTC) You need to calculate the barrier heigh with respect to the reactants. you also haven't calculated the gibbs energy of reaction.
Conclusion
This module shows different computational methods were useful to analyse a variety of pericyclic reactions. The transition states were predicted and the vibrations and energies were used to analyse the selectivity of products. However, factors like solvents and other species exist in real situation and were not included in the calculation, these effects need more calculations or real experiments to be found. In Conclusion, these methods are quite useful for chemist but should be used with caution because it is approximation and other factor could affect the result.
Reference
- ↑ CRC Handbook of chemistry and physics, 2005, 86th edition, pp. 9-19
- ↑ CRC Handbook of chemistry and physics, 2005, 86th edition, pp. 9-19
- ↑ http://www.ccdc.cam.ac.uk/products/csd/radii/table.php4 [Accessed 30/10/12]