Rep:Mod:xlt15
Introduction
All molecules in this wiki page are analyzed and calculated by using a quantum chemistry package called Gaussian. The molecule is first created in Gaussian and the point group of this molecule is checked to ensure that it is in the right symmetry. Optimisation is run on the molecule and all key informations is included in the report. "Item" table with converged values of forces and displacements from *.log file for each molecule is included to show that the molecule has been optimised. Vibration frequency analysis and atomic charges for each molecule are included as well in the report. Some interesting molecular orbitals are included for carbon monoxide and methane.
NH3 Molecule
Gaussian Calculation Summary
Gaussian calculation was done on NH3 molecule.
Calculation Method : RB3LYP
Basis Set : 6-31G(d.p)
Final Energy E(RB3LYP) : -56.55776873 a.u.
RMS Gradient Norm : 0.00000485 a.u.
Point Group : C3V
Bond Length of N-H: 1.01798 Angstroms (Å)
Bond Angle of H-N-H: 105.741 °
"Item" Table from *.log File
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986278D-10
Optimization completed.
-- Stationary point found.
Image of molecule
Ammonia molecule |
Click here for optimisation file.
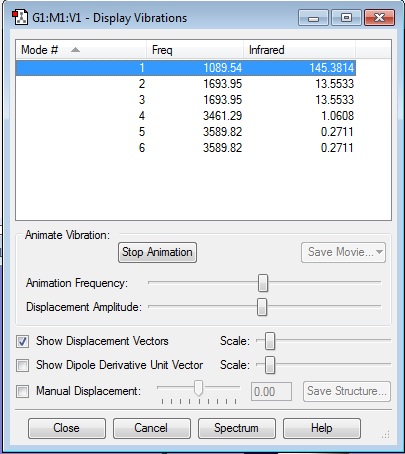
Display Vibration Window
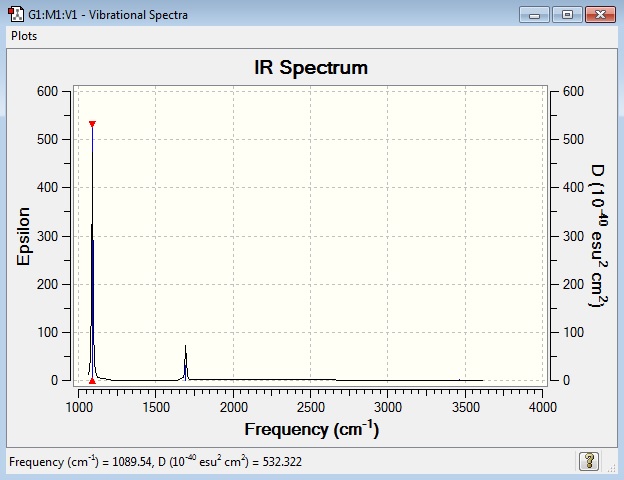
IR Spectrum
IR Analysis
According to 3N-6 rule, 6 modes are expected in NH3 molecule with N=4.
From the display vibration, it can be seen that mode 2 and mode 3 are degenerate because they have the same frequency and hence same energy. Also mode 5 and 6 are degenerate.
Mode 1, 2 and 3 are bending vibrations. Mode 4, 5 and 6 are stretches mode.
Mode 4 is highly symmetric.
Mode 1 is umbrella mode.
2 bands would be seen in experimental spectrum of gaseous ammonia because the IR intensities of mode 4, 5 and 6 are too low to be detected. There is no large change in dipole moment when molecule vibrates in these modes causing their intensities to be low. Mode 2 and 3 have the same frequency and so are observed as one band.
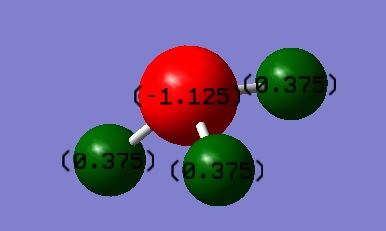
Atomic Charge
Charge on N = -1.125
Charge on H = 0.375
Nitrogen is more electronegative than hydrogen. Nitrogen attracts the bond pairs of electron towards itself, causing a negative charge build on it. Hydrogens are therefore electron deficient and have partial positive charge.
H2 Molecule
Gaussian Calculation Summary
Gaussian calculation was done on H2 molecule. Calculation Method : RB3LYP
Basis Set : 6-31G(d,p)
Final Energy E(RB3LYP) : -1.17853936 a.u.
RMS Gradient Norm : 0.00000017 a.u.
Point Group : D∞h
Bond Length of H-H : 0.74279 Angstroms (Å)
Bond Angle : 180 °
"Item" Table from *.log File
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
Image of molecule
Hydrogen molecule |
Click here for optimisation file.
Vibrational Frequency
Vibrational frequency of H2 is 4465.68 cm-1. H2 is not IR active because there is no change in dipole moment when molecule vibrates.
N2 Molecule
Gaussian Calculation Summary
Gaussian calculation was done on N2 molecule.
Calculation Method : RB3LYP
Basis Set : 6-31G(d,p)
E(RB3LYP) : -109.52359111 a.u.
RMS Gradient Norm : 0.02473091 a.u.
Point Group : D∞h
Bond Length of N-N Triple Bond: 1.10550 Angstroms (Å)
Bond Angle : 180 °
"Item" Table from *.log File
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.400996D-13
Optimization completed.
-- Stationary point found.
Image of molecule
Nitrogen molecule |
Click here for optimisation file.
Vibrational Frequency
Vibrational frequency of N2 is 2457.33 cm-1. N2 is IR inactive because there is no change in dipole moment when molecule vibrates.
Haber-Bosch process : Reaction between N2 and H2 forming NH3
Reaction equation: N2 + H2 -> 2NH3
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.11553746 a.u.
E(N2)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557907 a.u.= -146.82 kJ mol-1
Conclusion : The reaction is exothermic as heat energy is released (negative ΔE) and so the gaseous ammonia product is more stable.
Molecule of My Choice: Carbon Monoxide (CO)
Gaussian Calculation Summary
Gaussian calculation was done on CO molecule.
Calculation Method : RB3LYP
Basis Set : 6-31G(d,p)
Final Energy E(RB3LYP) : -113.30945314 a.u.
RMS Gradient Norm : 0.00000433 a.u.
Point Group : C∞V
Bond Length of CO Triple Bond : 1.13794 Angstrom (Å)
Bond Angle : 180 °
"Item" Table from *.log File
Item Value Threshold Converged?
Maximum Force 0.000007 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000003 0.001800 YES
RMS Displacement 0.000004 0.001200 YES
Predicted change in Energy=-2.221239D-11
Optimization completed.
-- Stationary point found.
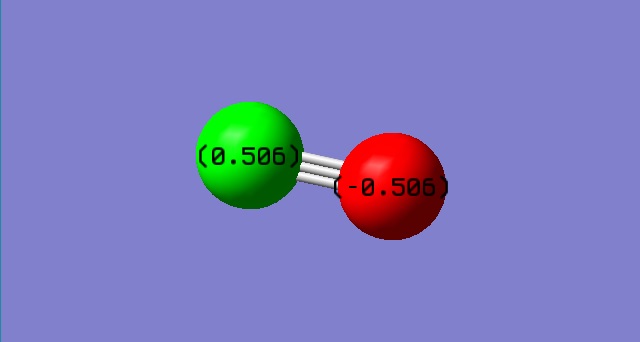
Image of molecule
CO molecule |
Click here for optimisation file.
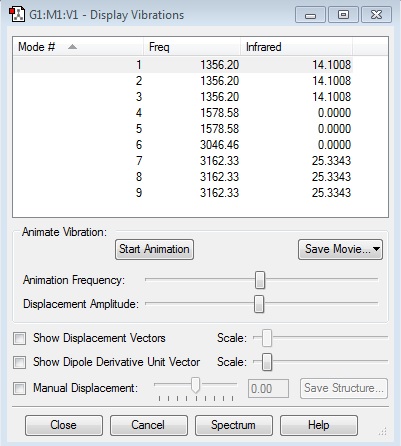
IR Analysis
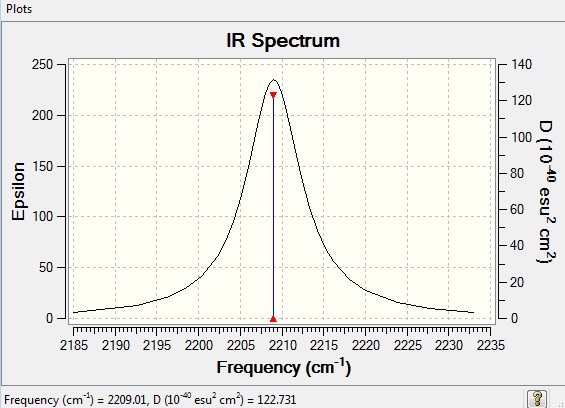
According to 3N-5 rule, 1 modes is expected in CO molecule with N=2. The vibrational frequency of CO molecule is 2209.01 cm-1. Stretching is the only vibrational mode as CO molecule is linear. It is IR active because there is a change in dipole moment when the molecule vibrates.
IR Spectrum
Atomic Charge
Charge on O = -0.506
Charge on C = 0.506
Oxygen is more electronegative than carbon. Oxygen attracts the bond pairs of electron towards itself, causing a negative charge build on it. Carbon is therefore electron deficient and have a partial positive charge.
5 Interesting Molecular Orbitals (MOs) of CO
Lowest Unoccupied Molecular Orbital (LUMO)

LUMO is the doubly degenerate pair of antibonding π orbitals .Size of MO indicates the contribution of each atomic orbitals of the atoms to the MO. 2p atomic orbitals of carbon and oxygen contribute to this MO. Carbon is less electronegative than oxygen so it has higher energy and so is closer in energy to LUMO. Larger lobes on carbon shows that they have more carbon character than that of oxygen. The relative energy of LUMO is -0.02178.
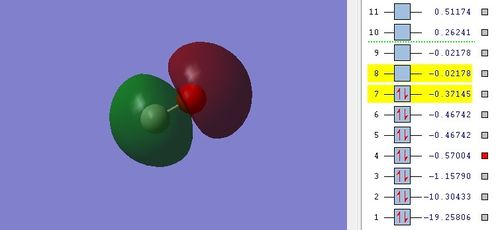
Highest Occupied Molecular Orbital (HOMO)

I think there is mixing between σ (2s) and σ (2p) MO and so 2s and 2p atomic orbitals of carbon and oxygen contribute to this MO as the lobe on carbon looks spherical. HOMO also has more contribution from carbon atom. Huge electron cloud on carbon atom shows that HOMO is located on carbon atom. The relative energy of HOMO is -0.37145. Large energy gap of 0.34967 is calculated from the value.
MO 3

There are two π bonding orbitals (1π in the MO diagram) which are of the same relative energy of -0.46742. Each 2p atomic orbitals from carbon and oxygen contribute to one π orbital by sideways overlapping. These MOs have the same shape and are perpendicular to each other.
MO 4
2s atomic orbitals of carbon and oxygen contribute to this MO by head-on overlap. The 2s AOs overlap out of phase so there is no electron density between the nuclei. The relative energy is -0.57004. The lobes on carbon and oxygen are of similar size and so the 2s AOs of carbon and oxygen contributed almost equally.
MO 5
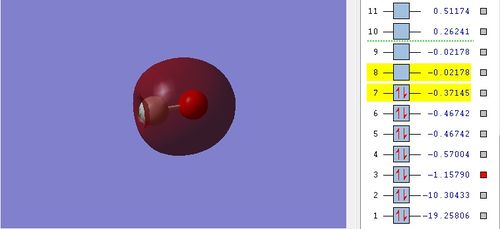
2s atomic orbitals of carbon and oxygen contribute to this MO by head-on overlap. Oxygen is more electronegative than carbon and has lower energy of 2s AO. The oxygen 2s AO contributes more to 2σ orbital than carbon 2s AO because energy level of 2s AO is closer to the 2σ orbital. The relative energy is -1.15790.
1σ and 1σ* molecular orbitals are at very much lower relative energies than the other MOs so they are essentially non-bonding.
Molecular Orbitals Diagram in Carbon Monoxide [1]
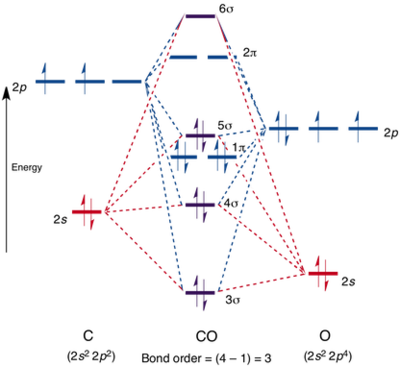
The MO diagrams show that there are s and p orbitals mixing because σ (2p) is at higher energy level than the degenerate π orbitals. HOMO which is 5σ (2p) in the MO diagram is actually non-bonding (lone pair on carbon atom). Orbitals of similar energy and symmetry can mix. 3σ (2s) has dominant oxygen 2s character, meaning it is non-bonding with respect to carbon-oxygen interaction.
Independence: CH4 Molecule
Gaussian Calculation Summary
Calculation Method : RB3LYP
Basis Set : 6-31G(d,p)
Final Energy E(RB3LYP) : -40.52401404 a.u.
RMS Gradient Norm : 0.00003263 a.u.
Point Group : Td
Bond length of C-H : 1.109197 Angstrom (Å)
Bond Angle of H-C-H : 109.471 °
"Item" Table from *.log File
Item Value Threshold Converged?
Maximum Force 0.000063 0.000450 YES
RMS Force 0.000034 0.000300 YES
Maximum Displacement 0.000179 0.001800 YES
RMS Displacement 0.000095 0.001200 YES
Predicted change in Energy=-2.256096D-08
Optimization completed.
-- Stationary point found.
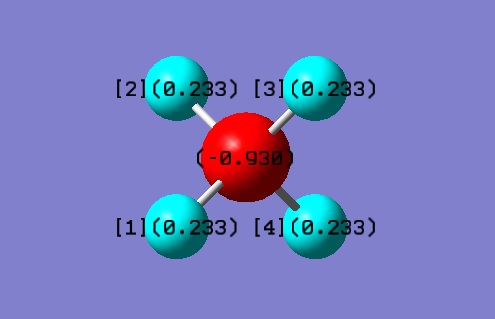
Image of molecule
Methane molecule |
Click here for optimisation file.
Display Vibration Window
IR Spectrum
IR Analysis
According to 3N-6 rule, 9 modes are expected in CH4 molecule with N=5.
From the display vibration, it can be seen that there are 3 sets of vibrational mode that are degenerate. Mode 1, 2 and 3 are degenerate because they vibrate at the same frequency. Mode 4 and 5 is another set of vibration that are degenerate and have the same energy. Also, mode 7, 8 and 9 are degenerate.
Mode 1, 2, 3, 4 and 5 are bending vibrations. Mode 6 is symmetrical stretch mode. Mode 7 and 8 are stretches mode. Mode 9 is asymmetrical stretch mode.
Only 2 bands at 1356.20 cm-1 and 3162.33 cm-1 would be seen in experimental IR spectrum of methane. The IR intensities of mode 4, 5 and 6 are not being detected because here is no change in dipole moment when molecule vibrates in these modes. Mode 1, 2 and 3 have the same frequency and so are observed as one band. Also mode 7, 8 and 9 have the same frequency and so are observed as one band.
sp3 Hybridization [2]

The valence electron configuration of carbon atom is 2s2 2px1 2py1. One of the 2s electron is promoted to 2pz and then these four atomic orbitals undergo hybridization to form 4 sp3 hybrid orbitals. Each of the sp3 hybrid orbital can then overlap with 1s orbital of hydrogen. Each of the C-H bond is therefore equivalent and has the same bond length.
Atomic Charge
Charge on C = -0.930
Charge on H = 0.233
Carbon is slightly more electronegative than hydrogen. Carbon attracts the bond pairs of electron towards itself, causing a negative charge build on it. Hydrogens are therefore electron deficient and have partial positive charge.
Molecular Obitals (MO)
A1 MO
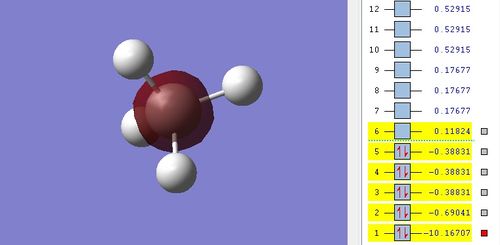
The two electrons from 1s atomic orbital (AO) of carbon form this MO and is therefore non-bonding orbital. It has the same energy as the 1s AO of carbon. 1σ MO is at very much lower energy than other MOs and its relative energy is -10.16707.
Bonding MO
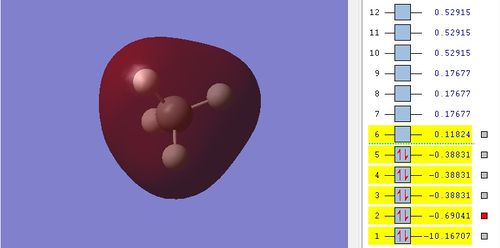
The 2s AO of carbon and 1s AO from each hydrogen atom overlap in-phase to form bonding orbital. The relative energy of MO is -0.69041.
T2 MO, HOMO (Highest Occupied Molecular Orbital)

There are three degenerate MOs which have relative energy of -0.38831. Each of the MO is contributed by 2p of carbon atom and 1s AO from each hydrogen atom overlap in-phase to form bonding orbital. These MOs are oriented in different directions and are perpendicular to each other.
Antibonding MO, LUMO (Lowest Unoccupied Molecular Orbital)

LUMO of methane has relative energy of 0.11824. 2s AO of carbon and 1s AO from each hydrogen atom by overlap out-of-phase to form antibonding orbital.
Reference
[1]. Molecular Orbitals Diagram of CO, http://www.chemtube3d.com/orbitalsCO.htm
[2]. sp3 Hybridization, http://www.chemicool.com/definition/hybridization.html
[3]. Chemistry: An Introduction to Organic, Inorganic and Physical Chemistry, Catherine E. Housecroft, Edwin C. Constable, Prentice Hall, 2010
[4]. Molecular Orbitals of CO, http://www.chemistry.uoguelph.ca/educmat/chm2060_preuss/L29-2013.pdf