Rep:Mod:xl9814
INTRODUCTION
This experiment is to use Gauss View to visualize the mechanism of different routes of three different reactions including Reaction of Butadiene with Ethylene,Reaction of Benzoquinone with Cyclopentadiene and Diels-Alder vs Cheletropic reaction. By analyzing the energies of products and calculating the activation energies and reaction energies, the route which is more preferred can be chosen.
Exercise 1
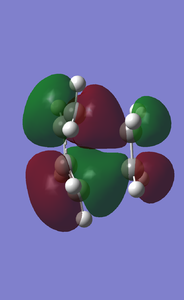
- MO Diagram
- MOs for the TS(with increasing energies)
- Ethene HOMO and LUMO diagram
- Butadiene HOMO and LUMO diagram
- Diagram of the reactants to measure bond lengths
- Diagram of the product to measure bond lengths
- Reaction can only happen when the molecules have the same symmetry(g and g, u adn u). The orbital overlap integral is zero for a gerade-ungerade interaction, non-zero for a gerade-gerade interaction and an ungerade-ungerade interaction.
- The C=C bond length in Butadiene and Ethene is 1.38Å. The C-C bond length in the Butadiene is 1.41Å. The C=C bond length in the product is 1.34Å, and the C-C bond length is 1.50Å(near the double bond) and 1.54Å(one bond away from the double bond). In the reaction, the double bond in Ethene and Butadiene becomes single bond and the single bond in butadiene becomes double bond. Therefore, The C-C bond length in Butadiene decreases when turning into products
as it changes to double bond in the product and the double bonds in Butadiene and Ethene increase when turning into products as they become single bonds in the product. The typical sp3 C-C bond length is 1.54Å and sp2 C-C bond length is 1.34Å.[1][2] The Van der Waals radius of the C atom is 1.85Å.[3] The length of the partly formed C-C bonds in the TS is 2.11Å which is longer.
Nf710 (talk) 23:08, 17 November 2016 (UTC) This is done pretty well. But you could have possible gone into more detail. you havent talk about how to find a TS or minimum. Or shown the vinration of stated if it syncronous or a syncronous
Exercise 2
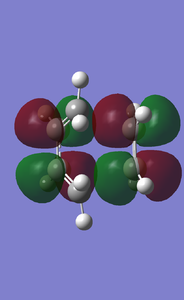
- Endo TS HOMO and LUMO diagram(with increasing energies)
- Exo TS HOMO and LUMO diagram(with increasing energies)
| Sum of electronic and thermal Free Energies | Endo | Exo |
|---|---|---|
| Reactants | 0.117201 | 0.117201 |
| TSs | 0.180251 | 0.181212 |
| Products | 0.109351 | 0.108077 |
- The kinetically more stable product is endo-product and the thermodynamically more favourable product is the exo-product.
- The activation energy of the reaction forming endo-product is 0.06305 KJ/mol(energy of TS - energy of reactants). The activation energy of the reaction forming exo-product is 0.064011 KJ/mol. The exo reaction has higher activation energy therefore it is the thermodynamically more favourable product. The endo reaction has lower activation energy so it is the kinetically more favourable product.
- The reaction energy of the endo reaction is -0.00785 KJ/mol( energy of products - energy of reactants) and is -0.009124 KJ/mol fot exo reaction.
- The energy of the exo-product is lower than the energy of the endo-product, so the exo-product is the thermodynamically more favourable one.
Nf710 (talk) 23:30, 17 November 2016 (UTC) I don't know how you have converted your energies or what. but they defiatly are not kJmol-1. Also you ahvent goven any reason why your exo and endo are the ordering you have given them. Exercise3
- gif for IRC of DielsAlder reaction(exo-product)
- gif for IRC of Cheletropic reactions
- gif for IRC of DielsAlder reaction(endo-product)
(This is not a DA reaction. Look at the bonds being formed - you are forming two C-O bonds Tam10 (talk) 14:46, 9 November 2016 (UTC))
| Sum of electronic and thermal Free Energies | DielsAlder(exo) | DielsAlder(endo) | Cheletropic |
|---|---|---|---|
| Reactants | 0.090179 | 0.084437 | 0.094723 |
| TSs | 0.092077 | 0.084684 | 0.099060 |
| Products | 0.067697 | 0.072297 | 0.067911 |
- The activation energy of the DielsAlder reaction is 0.001898 KJ/mol and is 0.004337 KJ/mol for the Cheletropic reaction.
- The reaction energy of the DielsAlder reaction is -0.022482 KJ/mol and is -0.031149 KJ/mol for the Cheletropic reaction.
(The energies from Gaussian are in Hartrees, with a conversion of 2625 kJ/mol to 1 Hartree. Tam10 (talk) 14:46, 9 November 2016 (UTC))
- The activaition energy of the Cheletropic reaction is higher, therfore the Cheletropic product is the thermodynamically more favourable product and the DielsAlder product is the kinetically more favourable product.
- The DielsAlder reaction is the preferred route as it has lower activation energy.
CONCLUSION
By using the first method, it can be seen that this method has lots of errors when running therefore it is not very reliable. Method 2 gives clear illustration of how the endo and exo products were formed by showing the mechanisms and helps to determine which of the products is more thermodynamically more favourable and which one is more kinetically favourable. Method 3 gives two different mechanisms for o-Xylylene and SO2 cycloaddition and by calculating the activation and reaction energies, it can be told which reaction mechanism is preferred. For method 2, it is clearly shown that the kinetically more stable product is endo-product and the thermodynamically more favourable product is the exo-product. For method 3, the DielsAlder reaction is the preferred route as it has lower activation energy.
(You haven't provided a reaction profile Tam10 (talk) 14:46, 9 November 2016 (UTC))
Reference
1.THE RELATION BETWEEN CARBON-HYDROGEN BOND LENGTHS AND BOND ANGLES BY C. W. N. CUMPER Woolwich Polytechnic, London, S.E. 18,Received 5th December, 1957
2.Handout #3, 5.12 Spring 2003, 2/12/03 Physical Properties: Bond Length, Bond Strength & Acidity
3.Van der Waals Radii of ElementsS. S. Batsanov,Center for High Dynamic Pressures, Mendeleevo, Solnechnogorskii raion, Moscow oblast, 141570 Russia