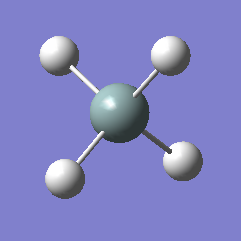
Rep:Mod:xQCsmash
Ammonia (NH3)
Opt N-H bond distance = 1.01798 ± 0.01 Å
Opt H-N-H bond angle = 105.741 ± 1°
For Ammonia
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986281D-10
| Molecule: | Ammonia (NH3) |
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(d,p) |
| Final energy E(RB3LYP): | -56.55776873 a.u. |
| RMS gradient: | 0.05399560 a.u. |
| Point group: | C3V |
Ammonia |
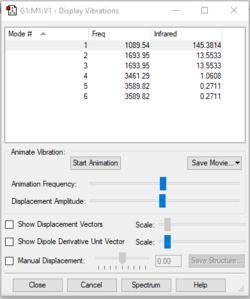
| Wavenumber (cm-1): | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry: | A1 | E | E | A1 | E | E |
| I.R Intensity: | 145 | 14 | 14 | 1 | 0 | 0 |
| Image: | 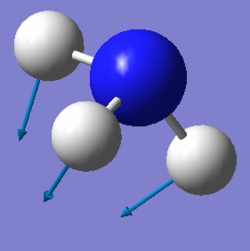 |
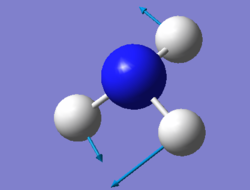 |
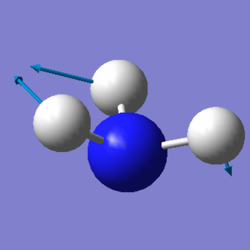 |
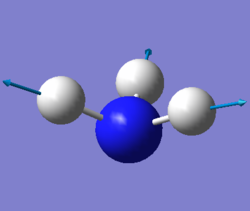 |
 |
|
We expected 6 modes of vibration following the nonlinear equation of 3N - 6, in which N is the number of atoms in the molecule. Modes with the same wavenumber are degenerate, as wavenumber directly correlates to energy. Both modes with a frequency of 1694 cm-1 are degenerate and 3590 cm-1 are also degenerate. Modes of 1090 cm-1 and both 1694 cm-1 are bending vibrations. All others are bond stretching vibrations. 1090 is the most symmetrical, having a C3 rotational axis and 3 mirror planes along each of the N-H bonds. 1090 is also known as the "umbrella" mode due to it's appearance as all 3 H atoms vibrate downwards relative to the nitrogen. In an experimental spectrum of gaseous ammonia, only 2 bands would be shown as 2 of the modes are degenerate and would only show up as a single peak. The rest have too low intensities to be detected.
Charge on nitrogen atom: -1.125
Charge on hydrogen atom: 0.375
It's expected for nitrogen to be more negative than hydrogen due to the electronegativity of nitrogen pulling the covalent electrons closer to itself.
Hydrogen (H2)
For Hydrogen
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
| Molecule: | Hydrogen (H2) |
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(d,p) |
| Final energy E(RB3LYP): | -1.17853936 a.u. |
| RMS gradient: | 0.00000017 a.u. |
| Point group: | D*H |
Hydrogen |
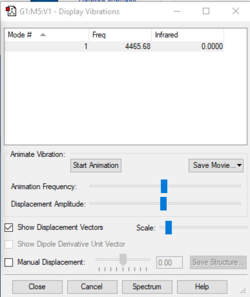
| Wavenumber (cm-1) | 4466 |
| Symmetry | SGG |
| I.R. Intensity | 0 |
| Image | 
|
NBO charge on both hydrogen atoms is 0
Nitrogen (N2)
For Nitrogen
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.400943D-13
| Molecule: | Nitrogen (N2) |
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(d,p) |
| Final energy E(RB3LYP): | -109.52412868 a.u. |
| RMS gradient: | 0.00000060 a.u. |
| Point group: | D*H |
Nitrogen |

| Wavenumber (cm-1) | 2457 |
| Symmetry | SGG |
| I.R. Intensity | 0 |
| Image | 
|
NBO charge on both nitrogen atoms is 0
N2 ligand associated complex DEKFUX
[[1]]
Optimised N-N bond length: 1.10550 Å
Bond length in transition metal complex: 1.086 Å
In the computed nitrogen molecule no other external factors were considered, so the bond length is for a molecule isolated from the environment. But the N2 ligand is subjected to the electron density from nearby groups and the transition metal it is bonded to. The packing of the molecules in the crystalline structure could force the bond length to be reduced compared to the isolated N2 molecule.
Haber-Bosch process
N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873
2*E(NH3)= -113.1155375
E(N2)= -109.52412868
E(H2)= -1.17853936
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074
Energy required for Haber-Bosch process: -146.5 kJmol-1
Ammonia is more stable than the constituent gases alone and is favoured in the reaction.
Silane (SiH4)
For silane
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-2.451071D-14
| Molecule: | Silane |
| Calculation method: | RB3LYP |
| Basis set: | 6-31G(d,p) |
| Final energy E(RB3LYP): | -291.88802760 a.u. |
| RMS gradient: | 0.00000002 a.u. |
| Point group: | TD |
Silane |
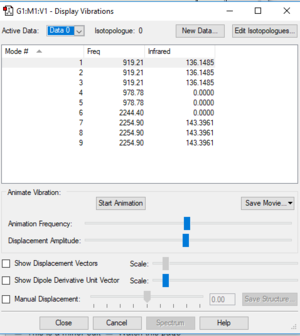
| Wavenumber (cm-1): | 919 | 919 | 919 | 979 | 979 | 2244 | 2255 | 2255 | 2255 |
| Symmetry: | T2 | T2 | T2 | E | E | A1 | T2 | T2 | T2 |
| IR intensity: | 136 | 136 | 136 | 0 | 0 | 0 | 143 | 143 | 143 |
| Image: | 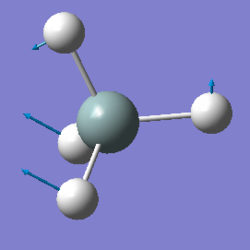 |
 |
 |
 |
 |
 |
 |
 |
|
NBO charge on Silicon is 0.336 while the charge is slightly negative on the hydrogens with -0.084
Marking
Note: All grades and comments are provisional and subjecct to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES, you could have explained that the charges are 0 as the electronegativities are equal.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 3.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
You have done a good job of presenting this information, well done! You should have explained the charges using an electronegativity argument. You should have analysed the computed vibrations. You identified most of the contributing AOs correctly! However, MO4 is not only build by the s orbitals of the Hs, the s orbital of Is is contributing as well. For the HOMO you underestimated the contribution of the p-orbital on Is. For the LUMO you stated 4 p orbitals contributing. The p orbitals of the Hs are not contributing at all to this! It actually is a combination of the s orbitals on the Hs and the p-orbital on Is
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
NO - No independent work has been identified.