Rep:Mod:winder24
Cyclopentadiene Dimerisation
Endo- vs Exo- Dimerisation
Despite both the endo- and exo- dimers of cyclopentadiene being theoretically possible, (Dimer - Exo and Dimer 2 - Endo), only the endo- isomer is formed.[1] Both isomers were modelled in Chembio 3D in order to discover whether this selectivity is the result of kinetic or thermodynamic factors.
When optimised in the MMFF94 force field, the energies were calculated as 41.27 for the endo- isomer and 55.38 for the exo- isomer. This shows that if thermodynamic factors dominated the reaction the exo- isomer would be formed as it has the lower energy, making it more thermodynamically stable; as the opposite is true, kinetics must be the dominating factor.
Hydrogenation of the Endo- Dimer
The two possible structures resulting from the hydrogenation of the endo- dimer are shown in Dimer 3 and Dimer 4 . The most thermodynamically stable is Isomer 4, which has a calculated energy of 41.3 kcal mol-1; this is 9.1 kcal mol-1 less than Isomer 3, which has a total energy of 50.4 kcal mol-1.
| Energy | Dimer 3 | Dimer 4 |
|---|---|---|
| Bond Stretching | 3.30 | 2.81 |
| Angle Bending | 31.9 | 24.7 |
| Stretch Bending | -2.09 | -1.65 |
| Torsional | -1.46 | -0.35 |
| Out of Plane Bending | 0.01 | 0.00 |
| Van Der Waals | 13.6 | 10.6 |
| Electrostatic Interactions | 5.11 | 5.15 |
| Total | 50.4 | 41.3 |
As the table of calculated energies shows, by far the greatest contribution to the energy comes from angle bending. This is unsurprising because the molecule comprises of a series of rings which require deviation from ideal bond angles in order to join together. Deviations from ideal Van der Vaals interactions also contribute to the total energy of the molecule, again because the atoms are locked in a multi-ring structure which forces them closer together than the sum of their Van der Waals radii; this causes repulsion.
The 6 membered ring ( containg the double bond in Isomer 3) is bridged by a carbon atom, this makes it more strained than the 5 membered ring (containing the double bond in Isomer 4). The double bond is shorter than a standard carbon bond and so strains the angles within the ring more than if it were a single bond. Isomer 3 has the double bond in the most strained of the two rings, where it has a greater disruptive effect. This is why the angle bending energy of Isomer 3 (31.9) is much greater than that of Isomer 4 (24.7) and the reason why Isomer 3 is the least stable. All other computed energies were similar, this reflects the fact that the only difference between the isomers is the position of the double bond and two hydrogen atoms.
Intermediate 9 and Intermediate 10 were both modelled in Chembio 3D using the MMFF94 force field to minimise the energy of the structures. The calculations showed that Intermediate 9 has an energy of 70.5 kcal mol-1 compared with 60.6 kcal mol-1 for Intermediate 10; Intermediate 10 is the most stable. The bond angles in the larger ring structure in Intermediate 9 are forced to deviate further from their optimal positions in order to accommodate for the carboxyl group facing upwards, it is this which increases the energy.
The functionalisation of the alkene in this structure has been found to proceed more slowly than expected, it is hyperstable [2]. Literature suggests that the hyperstability of the alkene is not due to steric hindrance or a particularly strong π-bond, but a result of the fact that the molecule would be more strained if a single bond and two functional groups were to replace the double bond. In order to test this, the product of hydrogenation of the alkene was modelled for both Intermediate 9 and Intermediate 10; these are Hydrogenated Intermediate 9 and Hydrogenated Intermediate 10. As literature suggested, both of these products had a higher energy than their corresponding alkene intermediate - shown in the table below.
| Molecule | Intermediate 9 | Intermediate 10 |
|---|---|---|
| With C=C | 70.5 | 60.6 |
| Hydrogenated | 81.8 | 71.5 |
These results show that the reason for the slow functionalisation of these intermediates is most likely because of the added strain in the ring when the double bond lengthens to become a single bond.
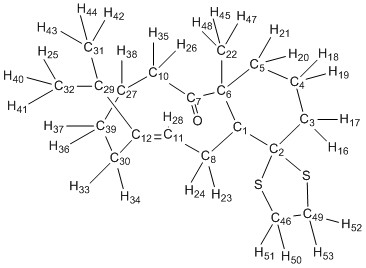
Predicting the NMR Spectra of Taxol Intermediate 18

Taxol Intermediate 18 was modelled in Avogadro; using the Gaussian Extension, the 13C and 1H NMR spectra were then calculated (B3LYP, 6-31G(d,p), Solvent = Chloroform). The results are tabulated below and the spectra (without methyl hydrogens averaged) are shown to the right.
| Integral | Calculated Shift (ppm) | Reported Shift (ppm) | Atom Number |
|---|---|---|---|
| 3H | 1.14 | 1.03 | 25,41,40 |
| 3H | 1.27 | 1.07 | 43,42,44 |
| 3H | 1.42 | 1.10 | 48,47,45 |
| 3H | 1.22, 1.36, 1.52 | (m, 1.20-1.5) | 18,20,21 |
| 1H | 1.58 | 1.58 | 35 |
| 6H | 1.85, 1.85, 2.00, 2.00, 2.28, 2.33, | (m, 1.70-2.20) | 17,37,33,38,16,19 |
| 4H | 2.48, 2.55, 2.61, 2.81 | (m, 2.35-2.70) | 36,13,34,26 |
| 6H | 2.81, 2.92, 2.92, 3.00, 3.13, 3.13 | (m, 2.70-3.00) | 52,51,53,50,23,24 |
| 1H | 5.99 | 5.21 | 28 |
The recorded literature spectra has several groups of multiplets, the result of many ring hydrogens in similar environments with complex coupling. Gaussian would take too long to calculate the couplings so this was not done. The values of chemical shifts match quite well with the literature spectrum, however this assignment seems too random. The alkene-hydrogen is furthest from its actual value, suggesting that the conformation here differs from the real structure.

| Integral | Calculated Shift (ppm) | Reported Shift (ppm) | Atom Number |
|---|---|---|---|
| C | 22.5 | 19.8 | 31 |
| C | 24.2 | 21.3 | 4 |
| C | 24.6 | 22.2 | 39 |
| C | 26.5 | 25.3 | 32 |
| C | 28.4 | 25.56 | 30 |
| C | 32.6 | 30.00 | 8 |
| C | 33.7 | 30.84 | 22 |
| C | 44.2 | 35.47 | 46 |
| C | 45.7 | 36.78 | 49 |
| C | 38.7 | 38.73 | 5 |
| C | 41.3 | 40.82 | 10 |
| C | 48.1 | 43.2 | 3 |
| C | 49.7 | 45.5 | 29 |
| C | 55.0 | 50.9 | 27 |
| C | 55.0 | 51.3 | 6 |
| C | 65.9 | 60.5 | 1 |
| C | 93.26 | 74.6 | 2 |
| C | 120 | 121 | 11 |
| C | 148 | 149 | 12 |
| C | 213 | 211 | 7 |
Most shifts match very closely with their predicted values, deviation is only over 5ppm in a few cases - those where the carbon atoms are attached to sulphur. This is most obvious for atom 2, which is 20ppm from the recorded value; atom 2 is connected to 2 sulphur atoms and would need correction in order to improve its accuracy. Atoms 49 and 46 are connected to 1 sulphur atom each so the deviation is not so large. As a rough estimate, I would suggest each sulphur attached to a carbon atom requires about a -10 ppm correction to the chemical shift.
Analysis of the Properties of Two Synthesised Alkene Epoxides
Objectives
The objective of this exercise is to predict and rationalise the structure of 4 alkene epoxides, two of which are synthesised using the Shi Catalyst and two using the Jacobsen Catalyst. The two alkene reagents are 1,2 Dihydronapthalene and Trans-Stilbene.
The Shi Catalyst
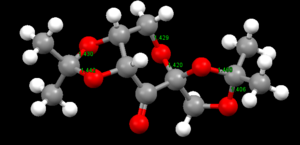
The Shi Catalyst has 3 C-O-C anomeric centres. The central anomeric centre has two bonds of roughly the same length (1.420, 1.429 angstroms)and is not of particular interest. However, the two outer anomeric centres have significant bond length asymmetry (1.442, 1.430 angstroms and 1.406, 1.460 angstroms). It is likely that this asymmetry improves the positioning of the methyl groups for sterically blocking access to the active site. The ways in which the incoming alkene can approach the dioxirane are limited by the steric bulk and position of the methyl groups and 5 membered rings, leading to high enantiomeric selectivity.
There are 8 conceivable transition states for the epoxidation of a trans-alkene using this catalyst. These were modelled using trans-β-methyl styrene in order to predict the most abundant isomer.[3][4][5][6][7][8][9][10]
| Transition State | Energy (Hartree) | Energy(kJ mol-1) |
|---|---|---|
| R,R Below-Exo | -1343.022 | -3526103 |
| R,R Below-Endo | -1343.019 | -3526095 |
| R,R Above-Exo | -1343.029 | -3526122 |
| R,R Above-Endo | -1343.032 | -3526130 |
| S,S Below-Exo | -1343.018 | -3526096 |
| S,S Below-Endo | -1343.016 | -3526088 |
| S,S Above-Exo | -1343.023 | -3526106 |
| S,S Above-Endo | -1343.025 | -3526112 |
The transition state which leads to the formation of the R,R enantiomer, with the molecule approaching the top oxygen in the endo conformation, has the lowest energy (R,R Above-Endo). This is in agreement with experimental results, which show the R,R configuration to be the most abundant. [11]
By matching the lowest energy transition states forming both enantiomers (R,R Above-Endo and S,S Above-Endo), the Gibbs free energy (ΔGTS) and equilibrium constant were calculated(ΔG=-RTln(K)) . ΔGTS=20.21 kJ mol-1 and K = 3501; this is a large value of K, suggesting that there should be a high enantiomeric excess (ee) of the R,R configuration. Using ΔG=RT ln((1+ee)/(1-ee)), the ee was calculated to be 99.9%; this is higher than experimentally determined values which suggest the ee is 94%. [12] There are many possible reasons for this difference, but solvent effects appear to contribute significantly; in CH3CH2CN, for example, the ee is as low as 80% for the same reaction.[12]
Jacobsen's Catalyst

The bulk of the t-butyl groups in Jacobsen's catalyst prevent a coordinating alkene from approaching easily from any direction but across the diimine. This makes it excellent for the epoxidation of cis- disubstituted alkenes; it is difficult for trans- alkenes to approach without steric interference as one R- group will always have to be facing into the existing ligand, but cis-disubstituted alkenes may approach with both R- groups facing away.[13]
There are 4 possible transition states for epoxidation of cis-alkenes using this catalyst. They have been modelled using cis-β-methyl styrene. [14][15][16][17]
| Transition State | Energy (Hartree) | Energy(kJ mol-1) |
|---|---|---|
| S,R Exo | -3383.251 | -8882724 |
| S,R Endo | -3383.250 | -8882723 |
| S,R Exo | -3383.260 | -8882747 |
| S,R Endo | -3383.253 | -8882731 |
The lowest energy transitions state for this catalyst is that in the S,R Exo conformation. The free energy of transtition between the favoured (S,R)and unfavoured (R,S) transition states (ΔGTS) =23.63 kJ mol-1 and K = 13871. The correct enantiomer (S,R) is predicted to be formed; the predicted ee is 100% (99.998%), which is again much higher than experimental results (92%)suggest.[13].
Calculated NMR of the Possible Epoxides
There are 4 possible epoxides which can be synthesised from 1,2 Dihydronapthalene and Trans-Stilbene the R,R or S,S enantiomers for Trans-Stilbene and the R,S or S,R enantiomers for 1,2 Dihydronapthalene. The NMR spectra have been calculated in Gaussian to help assign the spectra which will be recorded in the laboratory next week.
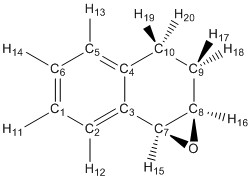
1,2 Dihydronaphthalene S,R[18]
1H NMR(CDCl3): δ 7.61 H12 (m,1H), δ 7.35 H11,14 (m,2H), δ 7.29 H12 (dd,1H), δ 3.57 H15 (d,j=4.4Hz, 1H), δ 3.49 H16 (ddd,j= 7.43 2.1 4.41 Hz, 1H), δ 2.91 H20 (ddd,j=17.5 6.19 3.60 Hz,1H), δ 2.34 H19 (ddd, j= 17.5 6.08 1.84 Hz,1H), δ 2.25 H18 (dddd, j= 15.94 7.43 6.19 1.84 Hz, 1H), δ 1.73 H17 (dddd, j= 15.94 6.08 3.60 2.1 Hz, 1H).
13C NMR(CDCl3):δ 152 C9, δ 151 C10, δ 128 C7, δ 126 C8, δ 51 C1, δ 50 C5, δ 48 C6, δ 42 C3, δ 35 C4,
Allowing for the effects of the aromatic ring changing the coupling on the aromatic hydrogen atoms and the resolution making some highly split overlapping shifts look like multiplets, the hydrogen NMR spectra shows similarity to spectra in literature [19]. The carbon NMR spectra shows little resemblance to literature as all shifts are more than 10ppm away from the recorded spectra.[19] This suggests that the optimisation may not have been completely successful or different basis set is required to improve the accuracy.
1,2 Dihydronaphthalene R,S[20]
The R,S and S, R molecules are enantiomers, they will have identical chemical shifts in NMR spectroscopy unless in a chiral solvent. The calculated shifts demonstrated this is the case, they were almost identical the the spectrum above.
Trans-Stilbene S,S[21]
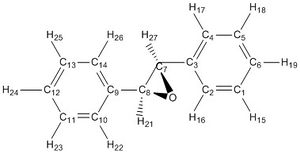
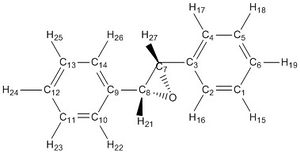
1H NMR(CDCl3): δ 7.50 H15,16,17,18,19,22,23,24 (m,10H), δ 3.57 'H21,27 (d,j=1.19 Hz,2H)
This is not a complex spectrum due to the high symmetry of the molecule. The predicted spectrum is a very good match for literature - δ 7.42-7.32 (m,10H) δ 3.87 (s,2H). [22]
Trans Stilbene R,R[23]
Again, due to the fact that the enantiomers have identical properties in an achiral environment, such as CDCl3, the spectrum of this compund will not differ from the S,S enantiomer; optical rotation is required to distinguish between them.
Calculated Optical Rotations of the Enantiomers[24][25][26][27]
| Enantiomer | Optical Rotation (o) | Literature |
|---|---|---|
| Trans-Stilbene S,S | -298 | -249 [28] |
| Trans Stilbene R,R | 297.92 | 250.8 [29] |
| 1,2 Dihydronapthalene S,R | 35.5 | -90.5[19] |
| 1,2 Dihydronapthalene R,S | 155.85 | 90.5 [19] |
Gaussian's calculation of the optical rotation was successful for both trans-stilbene enantiomers; both were within 500. This will allow me to correctly assign the epoxides which will be synthesised next week. 1,2 Dihydronapthalene was partially successful - the R,S enantiomer has a rotation of a similar magnitude to literature. The S,R enantiomer prediction failed to determine even the correct sign of the rotation, it should be negative. This is most likely the result of a slight difference in optimised structure, which can have a big effect on the rotation; in order to fix the problem, the molecule would have to be rearranged and re-optimised, due to time constraints this could not be done.
Non-Covelant Interactions in the Active Site
Orbital |
This diagram shows the non-covelant interactions (NCI) which exist in the lowest energy Shi catalysed epoxidation transition state. The large amount of green, and even some blue, shows there are many favourable interactions. Of particular interest are: the interaction between the hydrogen adjacent to the dioxirane and the phenyl ring of the incoming alkene; the interaction between the oxygen atoms in the upper 5 membered ring and the methyl end of the incoming alkene and The interaction between the lower oxygen of the dioxirane and the methyl group.
The first two of these interactions map the Van der Waals interaction between the catalyst and alkene. The fact that these interactions are quite strong for this transition state suggests part of the reason why its energy is lowest in this configuration despite the rather bulky phenyl group being in closer proximity to the catalyst than some other states.
The third of these interactions would be the same for all transition states. It is possible that this interaction makes it slightly more favourable for the methyl group to be in closer proximity to the lower oxygen, increasing steric repulsion between catalyst and alkene in the transition states with bonding to this oxygen.
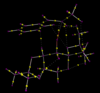
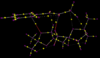
QTAIM Analysis
Similar two above, QTAIM analysis was performed, this time on the lowest energy R,R transition state and the lowest energy S,S transition state, for comparison. Both molecules had several bond critical points (BCP) which were not between two covalently bonded atoms. For the R,R transition state these were between the same atoms as shown above in NCI analysis.
For the S,S transition state there are several BCP's between hydrogen and oxygen atoms. In this molecule these would not be hydrogen bonds (the hydrogen atoms are not connected to sufficiently electronegative atoms), but would be Van der Waals interactions. There is also a Van der Waals interaction between the tertiary carbon of the phenyl ring and a hydrogen atom, this is similar to the R,R state and most likely also helps to lower the energy.
Possible Future Epoxides for Analysis
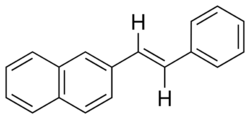
Although no epoxides could be found with an optical rotary power (ORP) of greater than 500 deg for which the corresponding alkene was readily avaliable, one possible candidate with a slightly lower ORP has been identified.
Trans-2-(naphthalen-2-yl)-3-phenyloxirane has a reported ORP of -244.9 deg, but has only been reported in one paper. Its similarity to several of the epoxides currently used increases the chance that its synthesis from trans-2-styryl naphthalene is possible. Trans-2-styryl naphthalene is readily avaliable from Sigma Aldrich (cat. no. R365246), which makes it an ideal candidate.
References
- ↑ Pierluigi Caramella , Paolo Quadrelli , and Lucio Toma , J. Am. Chem. Soc., 2002, 124, 1130-1131.DOI:10.1021/ja016622h
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.738028
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.749615
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.738036
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.738037
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.738038
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.739115
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.739116
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.739117
- ↑ Wong O A , WAng B , Zhao M X , Shi Y J. Org. Chem., 2009, 74, 6335-6338.DOI:10.1021/jo900739q
- ↑ 12.0 12.1 Zhi-Xian Wang , Yong Tu , Michael Frohn , Jian-Rong Zhang , and Yian Shi J Am. Chem. Soc, 1997,119, 11224-11235.DOI:10.1021/ja972272g
- ↑ 13.0 13.1 Eric N. Jacobsen , Wei Zhang , Alexander R. Muci ,James R. Ecker , Li Deng J Am. Chem. Soc, 1991,113, 7063-7064.DOI:10.1021/ja00018a068
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.740436
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.783851
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.740437
- ↑ Rzepa, Henry , Figshare, .DOI:10.6084/m9.figshare.783898
- ↑ Winder, Richard 2012, DOI:10042/25735
- ↑ 19.0 19.1 19.2 19.3 Donglu Xiong, Xiaoxue Hu, Shoufeng Wang, Cheng-Xia Miao, Chungu Xia, Wei Sun , European Journal of Organic Chemistry., 2011, 23, 4289-4292.DOI:10.1002/ejoc.201100512 Cite error: Invalid
<ref>tag; name "joc.201100512" defined multiple times with different content - ↑ Winder, Richard 2012, DOI:10042/25736
- ↑ Winder, Richard 2012, DOI:10042/25734
- ↑ Kerstin A. Stingl, Katharina M. Weiß,Svetlana B. Tsogoeva, Tetrahedron.,2012, 68, 8493-8501.DOI:10042/25734
- ↑ Winder, Richard 2012, DOI:HERE INSERT HERE
- ↑ Winder, Richard , 2013, DOI:10042/25785
- ↑ Winder, Richard , 2013, DOI:10042/25786
- ↑ Winder, Richard , 2013, DOI:10042/25787
- ↑ Winder, Richard , 2013,DOI:10042/25785
- ↑ Read John, Campbell, Ishbel J. Chem. Soc., 1930, 2377-2384.DOI:10.1039/JR9300002377
- ↑ David J. Fox, Daniel Sejer Pedersen, Asger B. Petersen and Stuart Warren Org. Biomol. Chem., 2006, 3117-3119.DOI:10.1039/B606881B
