Rep:Mod:wikifault
What is the molecule?
NH3
What is the calculation method?
RB3LYP
What is the basis set?
6-31G(d,p)
What is the final energy E(RB3LYP) in atomic units (au)?
-56.55776873
What is the RMS gradient (au)?
0.00000485
What is the point group of your molecule?
C3V
Optimised Bond Length (Å):
1.01798
Optimised Bond Angle:
105.741
Optimisation Data:
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmol Image:
NH3 |
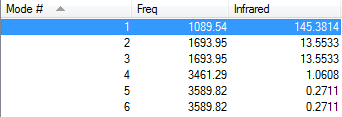
How many modes do you expect from the 3N-6 rule?
6
Which modes are degenerate (ie have the same energy)?
2,3;5,6
Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Bending Modes = 1,2,3
Stretching = 4,5,6
Which mode is highly symmetric?
4
One mode is known as the "umbrella" mode, which one is this?
1
How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
4
Charge for N = -1.125
Charge for H = 0.375
This is expected because nitrogen is more electronegative than hydrogen, hence I expect nitrogen to be negative and hydrogen to be positive.
N2 Molecule
The summary information
What is the molecule?
N2
What is the calculation method?
RB3LYP
What is the basis set?
6-31G(d,p)
What is the final energy E(RB3LYP) in atomic units (au)?
-109.52412868
What is the RMS gradient (au)?
0.00000060
What is the point group of your molecule?
D*H
Optimised Bond Length (Å):
1.10550
Optimised Bond Angle:
180
Optimisation Data: