Rep:Mod:wc1911
Part 1
The Hydrogenation of Cyclopentadiene Dimer
Introduction
At room temperature, Cyclopentadiene dimerises to produce dicyclopentadiene via Diels-Alder reaction. Specifically, the endo dimer 2 is preferred than the exo dimer 1. To explore the reason accounts for this phenomenon, both 1 and 2 were drew and optimized using MMFF94s force field in Avogadro. Also, hydrogenation of endo dimer can be done across two different bonds, which results in two different products 3 and 4.

exo and endo dimer
| molecules | 1(dimer exo) | 2(dimer endo) | |||||||
| structure |
|
|
|||||||
| Total bond stretching energy(kcal/mol) | 3.54303 | 3.46790 | |||||||
| Total angle bending energy(kcal/mol) | 30.77273 | 33.18940 | |||||||
| Total stretch bending energy(kcal/mol) | -2.04142 | -2.08218 | |||||||
| Total tortional energy(kcal/mol) | -2.73046 | -2.94949 | |||||||
| Total out-of-plane bending energy(kcal/mol) | 0.01477 | 0.02183 | |||||||
| Total Van Der Waals energy(kcal/mol) | 12.80111 | 12.35869 | |||||||
| Total electronic energy(kcal/mol) | 13.01366 | 14.18452 | |||||||
| Total energy(kcal/mol) | 55.37342 | 58.19067 |
analysis

As can be seen from the table above, endo dimer has a higher energy geometry than exo dimer, which means exo dimer is thermodynamically preferred product. But in fact, endo dimer is the preferred product, which means this dimerisation process should be under kinetic control via a more stable endo transition state. Orbitals overlap during endo transition state is shown on the right. To explain the difference of energies of exo and endo dimer, bond angles are labelled in the picture, since angle bending energy is the main resource of this difference. Compared to supposed angles(sp3 109.5 and sp2 120), endo dimer deviates more than exo does, which leads to higher total energy.
Hydrogenation products
| molecules | 3 | 4 | |||||||
| structure |
|
|
|||||||
| Total bond stretching energy(kcal/mol) | 3.31178 | 2.82313 | |||||||
| Total angle bending energy(kcal/mol) | 31.93329 | 24.68580 | |||||||
| Total stretch bending energy(kcal/mol) | -2.10224 | -1.65722 | |||||||
| Total tortional energy(kcal/mol) | -1.46857 | -0.37855 | |||||||
| Total out-of-plane bending energy(kcal/mol) | 0.01309 | 0.00028 | |||||||
| Total Van Der Waals energy(kcal/mol) | 13.63885 | 10.63704 | |||||||
| Total electronic energy(kcal/mol) | 5.11949 | 5.14701 | |||||||
| Total energy(kcal/mol) | 50.44569 | 41.25749 |
analysis
Same computational procedures are performed on 3 and 4 as 1 and 2. Molecule 3 has higher total energy than molecule 4 apparently, which can be explained by the large difference of their angle bonding energies. Obviously, from the labelled angles shown, molecule 3 angles deviates more. So if hydrogenation is under kinetic control, product 3 may dominates, while thermodynamic control will favor product 4 definitely. Reactant 2 transformation into products 3 and 4 should be thermodynamically favored as products are lower energy geometries.
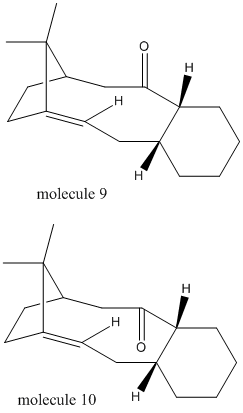
Intermediate 9 or 10 in the total synthesis of Taxol contains a carbonyl group pointing up or down, which can be converted to each other through bond rotation and so defined as Atropisomers. Using Avogadro MMFF94(s) force field to optimize both and to calculate energies. Compared to molecule 9, molecule 10 has lower angle bending energy due to less deviation from theoretical bond angle.(Table 4) Also, this low energy of molecule 10 contributes to the stability of it, which proved intolerance of high temperature under which slow decomposition of molecule 10 was observed.[1]
To explain the slow reactivity of intermediates 9 and 10, olefin strain energy is introduced, defined as the difference between the strain energy of an olefin and its corresponding saturated hydrocarbon(except carbonyl group). As can be seen from the table below, both 9 and 10 have lower energy than their parent molecules and this stability accounts for their relative inertness.[2]
| molecules | 9 | 10 | parent 9 | parent 10 | |||||||||||||
|
|
|
|
||||||||||||||
| Total bond stretching energy(kcal/mol) | 7.65562 | 7.73198 | 6.95860 | 6.86407 | |||||||||||||
| Total angle bending energy(kcal/mol) | 28.27693 | 19.60760 | 32.05834 | 25.84737 | |||||||||||||
| Total stretch bending energy(kcal/mol) | -0.08393 | -0.06128 | 0.30550 | 0.51634 | |||||||||||||
| Total tortional energy(kcal/mol) | 0.33595 | 3.23569 | 9.46216 | 10.93646 | |||||||||||||
| Total out-of-plane bending energy(kcal/mol) | 0.97742 | 0.86280 | 0.25479 | 0.09975 | |||||||||||||
| Total Van Der Waals energy(kcal/mol) | 33.08578 | 34.99921 | 32.72025 | 33.16216 | |||||||||||||
| Total electronic energy(kcal/mol) | 0.30592 | -0.04593 | -- | -- | |||||||||||||
| Total energy(kcal/mol) | 70.55371 | 66.33007 | 81.75963 | 77.42615 |
| carbons | angles supposed | 9 | 10 |
| 6,9,14 | 109.5 | 105 | 111 |
| 14,9,13 | 109.5 | 103 | 109 |
| 36,13,21 | 120 | 117 | 120 |
| 13.21.25 | 109.5 | 118 | 108 |
| 25.21.24 | 109.5 | 103 | 109 |
Spectroscopic Simulation using Quantum Mechanics

Using Avogadro to sketch and to optimize structures 17 and 18(MMFF94s), after which calculation of the geometry(17 chair) at the density functional level(DFT) is carried out by sending it to HPC system. The following work done is to compare this computational NMR spectrum with literature experimental one.
After optimization is carried out on molecule 17, it can be observed that the hexane ring of it has a chair form while a twisted boat form has been obtained on molecule 18. Adjusting the atoms positions of molecule 18 to get a lower energy chair form.
So I use the naturally occurred molecule 17 chair form to have NMR comparison and 18 chair form to compare the relative energies of these two isomeric configurations.
| molecules | 17 chair form | 18 chair form | ||||||
| structure |
|
| ||||||
| Energy | 439.461 kJ/mol | 420.574 kJ/mol |
Analysis
17 can be completely transformed into its conformational isomer 18 which possesses lower energy. Several bonds rotation must be done in this process and the most obvious change is the turn down of the pointing up carbonyl group in 17. This lower energy isomer 18, however, brings the methyl substituent(next to the carbonyl group) closer to the syn-oriented bridgehead methyl group, as shown on table above.[3]
Procedure using the HPC and analysis
| shift(ppm) | Degeneracy | Atoms | Reference values [3] | comparison |
| 5.1507207448 | 1.0000 | 17 | 'H NMR (300 MHz, CDCl3) δ4.84(1 H) ,3.40-3.10(m,4 H), 2.99(dd,1 H) ,2.80-1.35(series of m,14 H), 1.38(s,3 H), 1.25(s,3H),1.10(s,3 H),1.00-0.8(m,1 H) | 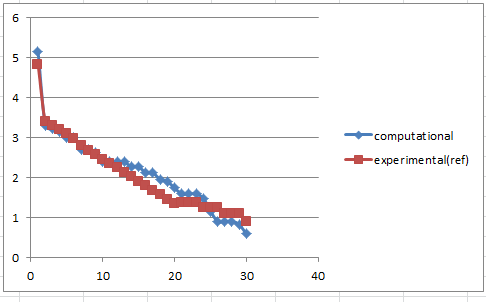
|
| 3.3080497824 | 1.0000 | 50 | ||
| 3.2207388624 | 1.0000 | 51 | ||
| 3.1592422462 | 1.0000 | 23 | ||
| 3.0146197695 | 2.0000 | 53,52 | ||
| 2.7156706265 | 2.0000 | 4,20 | ||
| 2.6367920939 | 1.0000 | 42 | ||
| 2.3970307659 | 4.0000 | 14,15,35,19 | ||
| 2.2736741818 | 2.0000 | 5,7 | ||
| 2.1222660004 | 2.0000 | 28,12 | ||
| 1.9542223978 | 1.0000 | 34 | ||
| 1.9034967023 | 1.0000 | 30 | ||
| 1.7457288626 | 1.0000 | 47 | ||
| 1.5957364246 | 3.0000 | 8,31,38 | ||
| 1.4893838736 | 1.0000 | 27 | ||
| 1.1633867526 | 1.0000 | 45 | ||
| 0.9073763124 | 3.0000 | 40,43,39 | ||
| 0.8236499126 | 1.0000 | 44 | ||
| 0.5920733176 | 1.0000 | 46 |
| shift(ppm) | Degeneracy | Atoms | Reference [3] | comparison |
| 216.1000554247 | 1.0000 | 13 | 13C NMR (75 MHz, CDCl3) ppm 218.79, 144.63, 125.33, 72.88, 56.19, 52.52, 48.50, 46.80, 45.76, 39.80, 38.81, 35.85, 32.66, 28.79, 28.29, 26.88, 25.66, 23.86, 20.96, 18.71 | 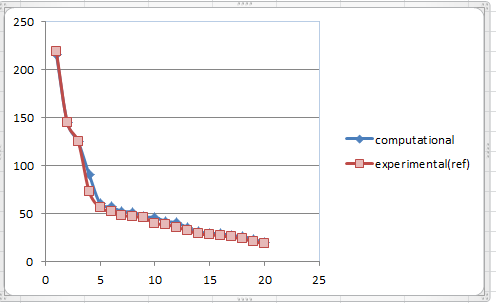
|
| 145.1230518451 | 1.0000 | 3 | ||
| 124.6989245528 | 1.0000 | 10 | ||
| 90.6460853480 | 1.0000 | 22 | ||
| 60.6391957007 | 1.0000 | 18 | ||
| 57.0551827243 | 1.0000 | 21 | ||
| 52.4718298107 | 1.0000 | 6 | ||
| 51.5473831103 | 1.0000 | 11 | ||
| 46.6913275278 | 1.0000 | 9 | ||
| 45.9547148608 | 1.0000 | 48 | ||
| 42.1758310396 | 1.0000 | 29 | ||
| 40.5782361033 | 1.0000 | 49 | ||
| 35.3211660520 | 1.0000 | 16 | ||
| 31.0114109626 | 1.0000 | 1 | ||
| 29.3385433737 | 2.0000 | 24,25 | ||
| 27.0963621866 | 1.0000 | 2 | ||
| 26.4009919833 | 1.0000 | 37 | ||
| 22.9019947936 | 1.0000 | 26 | ||
| 19.7261195422 | 1.0000 | 41 |
| molecule 17(H)chair | molecule 17(C) chair |

|

|
analysis
It can be seen from above scatter lines that 1H chemical shifts of experimental spectrum differs from the computational one, which may due to different conditions included in these spectra records(e.g. solvent, temperature, pressure.etc). The reason for the almost overlap lines of 13 C NMR is the scale of the diagram limits the reveal of deviations. However, deviations in both cases(1H and 13C) actually are small, which means the experimental data matches the computational one well.
| Type of energy(Hartree) | Molecule 17(chair) | Molecule 18(chair) |
| Zero-point correction | 0.468013 | 0.467883 |
| Thermal correction to Energy | 0.489499 | 0.489293 |
| Thermal correction to Enthalpy | 0.490443 | 0.490237 |
| Thermal correction to Gibbs Free Energy | 0.421225 | 0.421126 |
| Sum of electronic and zero-point Energies | -1651.414395 | -1651.417439 |
| Sum of electronic and thermal Energies | -1651.392910 | -1651.396030 |
| Sum of electronic and thermal Enthalpies | -1651.391965 | -1651.395086 |
| Sum of electronic and thermal Free Energies | -1651.461183 | -1651.464197 |
analysis
It can be concluded from both Table 4 Energy values and Table 1 Energy values that, molecule 18(in which six carbons ring takes chair form) is lower in terms of energy. But to transform 17 to 18,re-flux is needed to make this transformation done completely [3], which may due to high activation energy needed to rotate bonds. However, as 18 is lower in energy, complete transformation can be observed.
Part 2
Shi Fructose catalyst
Species 21 is a stable precursor to the Shi Fructose catalyst. [4] '[5]

| Bond assignment | atoms | bond length |
| a | 1O-2C | 1.420 А |
| b | 3O-2C | 1.405 А |
| c | 3O-4C | 1.460 А |
| d | 5O-4C | 1.406 А |
| e | 6O-7C | 1.442 А |
| f | 8O-7C | 1.430 А |
2 |
analysis
Typical bond length of C-O bond is 1.43 А. As can be seen from the Table 5, both bond a and b are shorter than the typical bond length, which can be explained by orbital overlaps between these bonds and the carbonyl π* bond. The torsional angle betwwen bond a and carbonyl group is 114 and the angle between b and carbonyl group is 123, both allow overlap well. Both the lone pairs shown on the picture can be donated to bond a or bond b, which is allowed by the axial position. So the reason accounts for the longer bond length of a than b is 3O connected to tertiary 4C donates lp more, which shortens bond b but lengthens bond a.
As 3O donates much electron density into interactions mentioned above,its ability of donating the other lp on it to bond d is weaker than donation of 5O to bond c, which makes bond c longer than d apparently.
The structure of 21 doesn't allow the orbital overlap between 6O-C and the carbonyl group because the Torsional Angle is 20.84 which is not good. But due to the inductive effect drawn, lp on 6O donates less than lp on 8O does, which leads to longer bond e than f.
The Jacobsen epoxidation catalyst[6]

2 |
structure analysis
In typical organic compounds, maximum attraction occurs at the distances 2.4 А for typical pairs of non-bonded H---H atoms and repulsion dominates when the distance < 2.1 А. Three of the distances measured above is attractive while the other one is repulsive interaction and this contributes to overall stabilization of the molecule. An interesting thing can be noted is that the Mn center adopts square pyramid structure other than trigonal bi-pyramid structure. Because four donor groups (except Cl)are on the same plane in sq.py structure, which avoids large torsional strain as in trigonal bi-pyramid, this structure is the preferred low energy structure.
Epoxidation
| molecules | β-methyl strene oxide(RR) | β-methyl strene oxide(SS) | trans-Stilbene oxide(RR) | trans-Stilbene oxide(SS) | ||||||||||||
| structure |
|
|
|
| ||||||||||||
| Energy(kJ/mol) | 96.8030 | 96.8002 | 165.198 | 165.211 |
| 1H β-methyl styrene oxide | 13C β-methyl styrene oxide | 1H Stilbene oxide | 13C Stilbene oxide |

|

|

| |

|

|

|

|
Assigning the absolute configuration of the product(ORP)
| Literature(2RR) | ORP(2RR) | Literature(2SS) | ORP(2SS) | |
| condition | 0.32g/100ml (concentration) ,90%ee, CHCl3(solvent), 250C, 589nm | CAM-B3LYP/6-311++g(2df,p),polar(optrot),scrf(cpcm,solvent=chloroform) | 1g/100ml (concentration) ,99%ee, CHCl3(solvent), 250C, 589nm | CAM-B3LYP/6-311++g(2df,p),polar(optrot),scrf(cpcm,solvent=chloroform) |
| Rotation | 44.3 deg [7] | 46.79 deg |
-41.8 deg [8] |
-47.77 deg |
| Literature(3RR) | ORP(3RR) | Literature(3SS) | ORP(3SS) | |
| condition | 0.73g/100ml (concentration) ,97%ee, CHCl3(solvent), 250C, 589nm | CAM-B3LYP/6-311++g(2df,p),polar(optrot),scrf(cpcm,solvent=chloroform) | 0.56g/100ml (concentration) ,89%ee, CHCl3(solvent), 200C, 589nm | CAM-B3LYP/6-311++g(2df,p),polar(optrot),scrf(cpcm,solvent=chloroform) |
| Rotation | 334.6 deg [9] | 297.7 deg |
-205.2 deg [10] |
-298.24 deg |
analysis
It can be noted from the table above that enantiomers of same molecule give out similar value of the optical rotations but opposite sign. Also, the computational value calculated are close to the literature result, although deviations exist. It can be concluded that the computational method used is reasonable and the literature records are correct.
Assigning the absolute configuration of the product(VCD)
| 2RR | 2SS |

|

|
| 3RR | 3SS |

|

|
analysis
It can be observed obviously that enantiomers have complete reverse VCD spectra(reflect along x-axis), which means the vibrational circular dichroism(VCD) should be useful in assigning absolute configuration. However, the appropriate instrument is not available in the department. It should be mentioned another technique can be used in identifying absolute configuration, the electronic circular dichroism(ECD), which is the UV/Vis spectrum recorded with polarised light. Although ECD is extremely useful in assigning absolute configurations always, absence of chromophore in the epoxides makes ECD useless here.
Using the Transition States Properties for the reaction
Introduction
As expoxidation products of same molecule can be enantiomeric and energies are different between these products, selectivity should exist and the enantiomeric excess can be computational calculated by considering energies of TS. Then this computational result can be used to check the enantiomeric assignment obtained before which is done by comparing computed and calculated optical rotations. Equation used in calculation is ΔG(TS)=-RTlnK and K is the equilibrium constant.
Energies obtained from given data and calculation
| data given | 2RR | 2SS | 3RR | 3SS |
| 1 | -1343.022970 | -1343.017942 | -1534.687808 | -1534.683440 |
| 2 | -1343.019233 | -1343.015603 | -1534.687252 | -1534.685089 |
| 3 | -1343.029272 | -1343.023766 | -1534.700037 | -1534.693818 |
| 4 | -1343.032433 | -1343.024742 | -1534.699901 | -1534.691858 |
| average | -1343.02598 | -1343.020513 | -1534.69375 | -1534.688551 |
| Energy difference | 0.005467 | 0.005199 | ||
| equi constant K | 328.13 | 247 | ||
| Enantiomer excess | 99.7% | 99.6% | ||
analysis
It can be concluded that in both cases,molecule 2(β-methyl styrene oxide) or 3(tans-Stilbene oxide), RR species are lower energy than SS species, which indicates that amount of RR overrides SS and that enantiomeric excess are calculated above. However, the enantiomeric values calculated are too high which are not same as experimental measured values(Table 8); it can be explained by the real conditions involved in experiments, which were not considered in computational calculation, such as temperature,solvents used and pressure, etc.
Investigating the non-covalent interactions in the active-site of the reaction transition state
| 2RR | 2SS |

|

|
Analysis
Pictures shown above reveal the non-covalent interactions, which include electrostatic attractions, hydrogen bonds and dispersion-like close approaches of pair of atoms. There are colorful rings in both RR and SS, which represent bond forming interaction there, while green areas mean mildly attraction. It may be seen from the pictures that the arrangement and orientation of atoms in RR transition state give out a larger green area than SS TS, which means RR TS should be more stable due to stronger attraction. It is where stereo-selectivity originating.
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
Blue arrow shown above indicates a BCP(bond critical point) which is associated with a covalent bond(forming new C--O). At the same time, two red arrows indicate two BCP which is associated with a non-covalent bond, which form between O(on 5 member ring the catalyst) and H connected to the reacting carbon center. This non-covalent bonds definitely orient where H points to and orient where epoxidation carries out, which makes sure RR selectivity to some extend.
Suggesting new candidates for investigations


|

| |
| conditions | c=0.03, solvent(ethanol),324 nm, 250C | c=0.03, solvent(ethanol),327 nm, 250C |
| OR | 853.9 deg | -1177.9 deg |
The starting material for the products shown above is Pulegone and the products are synthetically accessible, which can be done by mCPBA[12].
References
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
- ↑ 3.0 3.1 3.2 3.3 Spectroscopic data: L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283. DOI:10.1021/ja00157a043
- ↑ A. Burke , P. Dillon , Kyle Martin and T. W. Hanks,"Catalytic Asymmetric Epoxidation Using a Fructose-Derived Catalyst", J. Chem. Educ., 2000, 77, 271; DOI:10.1021/ed077p271
- ↑ O. A. Wong , B. Wang , M-X Zhao and Y. Shi J. Org. Chem., 2009, 74, 335–6338; DOI:10.1021/jo900739q
- ↑ J. Hanson,J. Chem. Educ., 2001, 78, 1266; DOI:10.1021/ed078p1266
- ↑ Wong,O.Andrea;Wang,Bin ;Zhao,Mei-Xin and Shi,Yian,Journal of Organic Chemistry, 2009, 74, 6335-6338. DOI:10.1021/jo900739q
- ↑ Lin,Hui;Liu,Yan;Wu,Zhong-Liu,Tetrahedron:Asymmetry, 2011, 22, 134-137.DOI:10.1016/j.tetasy.2010.12.022
- ↑ Wong,O.Andrea;Wang,Bin ;Zhao,Mei-Xin and Shi,Yian,Journal of Organic Chemistry, 2009, 74, 6335-6338. DOI:10.1021/jo900739q
- ↑ Niwa,Takashi and Nakada,Masahisa;Journal of the American Chemistry, 2012, 134, 13538-13541. DOI:10.1021/ja304219s
- ↑ Reusch;Johnson,Journal of Organic Chemistry, 1963, 28, 2557. DOI:10.1021/jo01045a016
- ↑ Adam,Waldemar;Paredes,Rodrigo;Smerz,Alexander K;Veloza,L.Angela,Liebigs Annalen/Recueil, 1997, 3, 547-552. DOI:10.1002/jlac.199719970316


