Rep:Mod:usagiphysical
The Cope Rearrangement of 1,5-hexadiene
1,5-hexadiene undergoes [3,3]-sigmatropioc rearrangement reaction as shown in Figure 1. For a long time its actual mechanism was the subject of some controversy and was studied by a large number of experimental and computational researches, but it is recently believed that this reaction is a concerted reaction via either a 'chair' or 'boat' conformation. The transition state with a 'boat' conformation is believed to be higher in energy than that with the 'chair' conformation. The objectives of this exercise are to locate the low-energy minima and transition structures on the 1,5-hexadiene potential energy surface by Gaussian calculation, in order to determine the preferred reaction mechanism.

Optimizing the Reactants and Products
Optimization via HF/3-21G
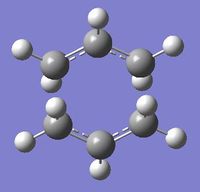
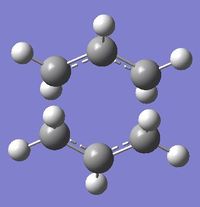
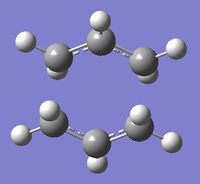
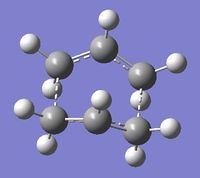
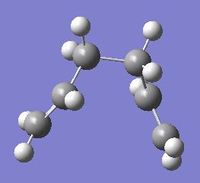
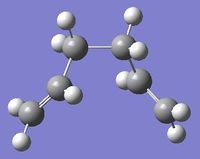
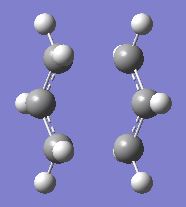
Four conformers (2 with "anti" linkage and 2 with "gauche" linkage) are 1,5-hexadiene were optimized and were confirmed to be anti2, anti4, gauche1 and gauche3 in Appendix 1 by matching the energies.
| Name | anti2 (Ci) | anti4 (C1) | gauche1 (C2) | gauche3 (C1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
|
| ||||||||||||
| Calculation Type | FOPT | FOPT | FOPT | FOPT | ||||||||||||
| Calculation Method | RHF | RHF | RHF | RHF | ||||||||||||
| Basis Set | 3-21G | 3-21G | 3-21G | 3-21G | ||||||||||||
| Point Group | Ci | C1 | C2 | C1 | ||||||||||||
| Energy/Ha | -231.69254 | -231.69097 | -231.68772 | -231.69266 | ||||||||||||
| .log file |
Optimization via B3LYP/6-31G*
The four comformers were then reoptimized at B3LYP/6-31G*.
| Name | anti2 (Ci) | anti4 (C1) | gauche1 (C2) | gauche3 (C1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
|
| ||||||||||||
| Calculation Type | FOPT | FOPT | FOPT | FOPT | ||||||||||||
| Calculation Method | RB3LYP | RB3LYP | RB3LYP | RB3LYP | ||||||||||||
| Basis Set | 6-31G* | 6-31G* | 6-31G* | 6-31G* | ||||||||||||
| Point Group | Ci | C1 | C2 | C1 | ||||||||||||
| Energy/Ha | -234.61071 | -234.61079 | -234.60786 | -234.61133 | ||||||||||||
| .log file |
Optimizing at B3LYP/6-31G* level of theory would add polarisation to atoms and improve the modelling of core electrons, producing more accurate description of orbitals as a result.[1]
Summary of Results and Discussion
| Structure | Point Group | Energy 3-21G (Ha) | Energy 6-31G* (Ha) | Sum of electronic and zero-point Energies (Ha) | Sum of electronic and thermal Energies (Ha) | Sum of electronic and thermal Enthalpies (Ha) | Sum of electronic and thermal Free Energies (Ha) |
|---|---|---|---|---|---|---|---|
| anti2 | Ci | -231.69254 | -234.61071 | -234.41613 | -234.40864 | -234.407694 | -234.45061 |
| anti4 | C1 | -231.69097 | -234.61079 | -234.42592 | -234.44740 | -234.44646 | -234.48194 |
| gauche1 | C2 | -231.68772 | -234.60786 | -234.46522 | -234.45810 | -234.45715 | -234.49541 |
| gauche3 | C1 | -231.69266 | -234.61133 | -234.46869 | -234.46146 | -234.46052 | -234.50011 |
log files: File:FREQ ANTI 2 631GD.LOG, File:ANTI4-631G FREQ.LOG, File:GAUCHE1-631G FREQ.LOG, File:GAUCHE3-631G FREQ.LOG
Based on the information in the tables above, the HF/3-21G and B3LYP/6-31G* basis set produced conformers with same point group.
The 'anti' conformers were expected to be more stable than the 'gauche' ones because of APP orbital interactions and steric repulsions. πC-C is a higher energy donor than σC-H, therefore the πC-C interacts better with the π*C-C app. Hence APP arrangement of the two vinyl groups is favorable. However unexpectedly the most stable conformer among the four is gauche3, as it is the conformation which possesses the lowest energy. Anti2 is more stable than anti4 and gauche3 is more stable than gauche1 because the two vinyl groups are further apart from each other.
Geometry Discussion

| Bond | HF/3-21G (Å ) | B3LYP/6-31G* (Å ) | Literature [2] | Angle | HF/3-21G | B3LYP/6-31G* | Literature [3] |
|---|---|---|---|---|---|---|---|
| C1-C2, C5-C6 | 1.323 | 1.334 | 1.3412 | C1-C2-C3, C4-C5-C6 | 124.8 | 121.8 | 122.5 |
| C2-C3, C4-C5 | 1.514 | 1.504 | 1.5077 | C2-C3-C4, C3-C4-C5 | 111.3 | 112.7 | 111.0 |
| C3-C4 | 1.548 | 1.548 | 1.5362 | C3-C2-H | 119.7 | 119.00 | 118.4 |
| C-H | 1.075 | 1.100 | 1.108 | C2-C3-C4-C5 | -179.989 | -180.000 | -178.3 |
It could be concluded that B3LYP/6-31G* was more accurate than the HF/3-21G as the bond length and angles were closer to the literature values.
Optimizing the "Chair" and "Boat" Transition Structures
The "Chair" and "Boat" Transition State
An allyl fragment was optimized at 'HF/3-21G(Figure 4), then two of these fragements were used to assemble the "chair" transition state with the terminal ends of the fragments 2.2Å apart (Figure 5). This "chair" structure was then optimised by a various methods i.e. Hessian and Frozen coordinates.

For the "boat" transition state, the QST2 method was used. In order to build a "boat" structure, all the atoms of the reactant and the product were numbered as shown in Figure 6.
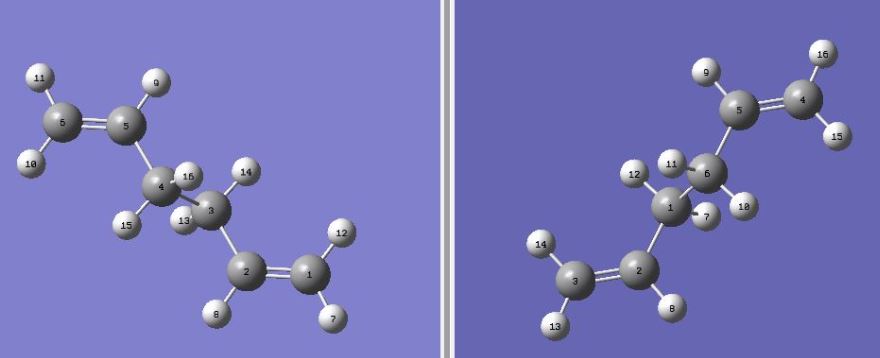
Figure 6
In order to assemble molecules into the desired boat form. The central C-C-C-C dihedral angeles (C2-5 for the reactant, C2-C1-C6-C5 for the product) of both molecules were modified from 180° to 0° and the C-C-C angles (C2-C3-C4 & C3-C4-C5 for the reactant, C2-C1-C6 & C1-C6-C5 for the product) were reduced from 113° to 100°.
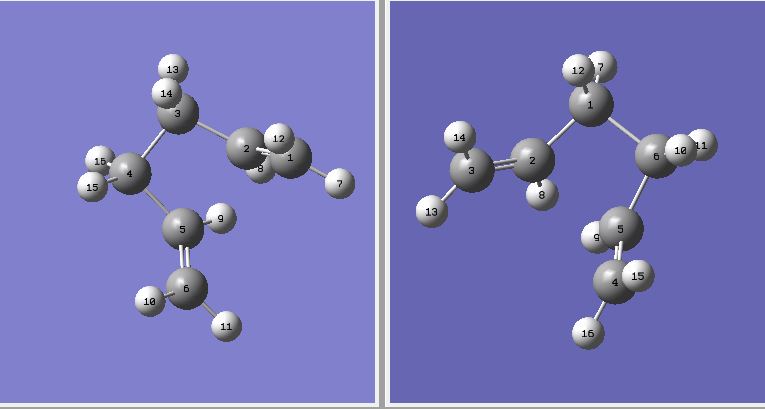
Figure 7 The resultant geometries of the reactant (left) and the product (right) after modification.
These were then optimized at HF/3-21G using the QST2 method. The resultant structure in shown in Table 5.
***Please click the links provided below to see the original file of Figure 8 and Figure 9 for the animation***
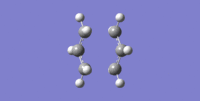
As seen from Figure 8, the Hessian method gives an imaginary frequency of 817.97cm-1 and the vibration mode corresponding to the Cope rearrangement. Both Hessian and the frozen coordinate methods give the tranistion bond lengths of about 2.02Å because of the reasonable assumption of the transition structure. For a molecule which is more complex, it will be more difficult to predict its transition structure by the Hessian method hence the frozen coordinate method would be preferable.
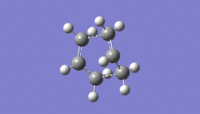
QST2 method gives an imaginary frequency of 839.94cm-1.
Intrinsic Reaction Coordinate
IRC for the chair transition state was computed on the HF/3-21G basis set, the reaction coordinate was only computed in the forward direction because it is symmetrical. The force constant setting was set to 'calculate always' and the number of points along the IRC is set to 50.
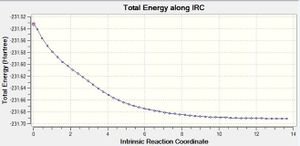
Figure 10. Initial IRC plot
From the structure we got from the initial IRC, it is clear that the transition state has not reached to its minimum as neither its energy nor structure corresponds to any of the conformers listed in Appendix 1. Hence the last point of the initial IRC was optimised to proceed further. The energy of the optimized structure (-231.69166699a.u) matches with the energy of gauche2 in Appendix 1. The IRC method suggests that gauche2 is the conformer of 1,5-hexadiene that leads to the chair transition state structure in the Cope Rearrangement of 1,5-hexadiene.
Reoptimization of Chair and Boat Transition States
The transition states were reoptimized at B3LYP/6-31G*
Activation Energies
| HF/3-21G | HF/6-31G* | |||||||
|---|---|---|---|---|---|---|---|---|
| Electronic Energy | Sum of Electronic and Zero-point Energies | Sum of Electronic and Thermal Energies | .log file | Electronic Energy | Sum of Electronic and Zero-point Energies | Sum of Electronic and Thermal Energies | .log file | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |||||
| Chair T.S. | -231.619322 | -231.466709 | -231.461351 | -234.556983 | -234.414919 | -234.408998 | ||
| Boat T.S. | -231.602802 | -231.450929 | -231.445301 | -234.543093 | -234.402338 | -234.396004 | ||
| Reactant (anti2) | -231.692535 | -231.539537 | -231.532565 | -234.611710 | -234.469202 | -234.461856 | ||
- 1Ha = 627.509 kcal/mol
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt. | |
|---|---|---|---|---|---|
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | at 0 K | |
| ΔE (Chair) | 45.70 | 44.70 | 34.07 | 33.16 | 33.5 ± 0.5 |
| ΔE (Boat) | 55.60 | 54.76 | 41.95 | 41.32 | 44.7 ± 2.0 |
The Diels Alder Cycloaddtion
Cis Butadiene
| Structure |
 |
| HOMO | 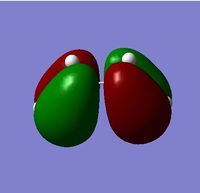 |
| LUMO | 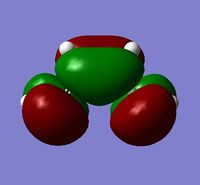 |
| Calculation Type | FOPT |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Point Group | C2V |
| Energy/Ha | 0.04879719 |
| .log file |
The Transition State of prototype reaction between ethylene and butadiene
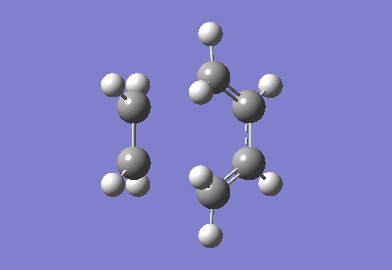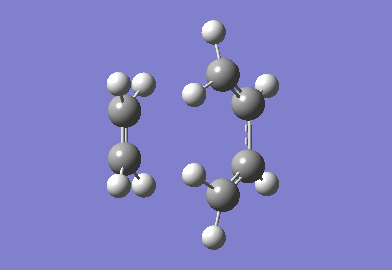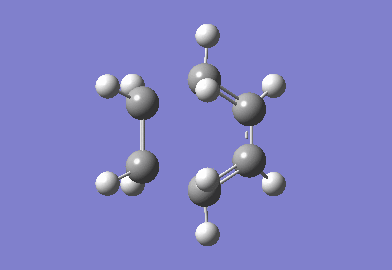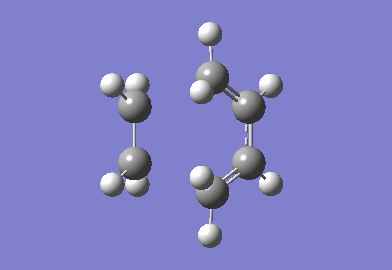
The cyclohexa-1,3-diene reaction with maleic anhydride
Further work
Reference
- ↑ Nigerian Journal of Chemical Research, 2007, 12. DOI:10.4314/njcr.v12i1.
- ↑ I. H. Gyorgy Schultz, Journal of Molecular Structure, 1994, 346, 63-69.
- ↑ I. H. Gyorgy Schultz, Journal of Molecular Structure, 1994, 346, 63-69.