Rep:Mod:uhlik414
Transition States Introduction
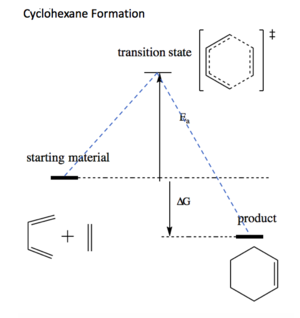
Transition state is a saddle point on the potential energy surface. Both the transition state (further referred as to TS) and local minima (equilibrium configurations) have first derivative (the gradient) equal zero, but the second derivative (curvature) for TS is negative, as it is the maximum on the potential energy curve, whereas (local) minimum will always have a positive curvature (configurations of molecule that 'sits' in a potential energy well). The initial structure of the TS has to be guessed, there is no guarantee that the correct conformation was found until further analysis is conducted. The structure of TS can be confirmed by frequency check and IRC (Intrinsic Reaction Coordinate) calculation. For a TS structure, one negative vibrational mode is expected as all other degrees of freedom (normal vibrational modes) given by the formula 3N-5 (for linear molecules) and 3N-6 (for non-linear molecules) are optimised to minimum (hence positive frequency). A negative frequency therefore indicates a presence of energy maximum i.e. transition state. The objective of this experiment was to analyse Diels-Alder [4+2] cycloaddition reaction profiles. Gaussian software was used to visualise and compute transition states and reactants/product energies and molecular orbitals. Three different reactions were under investigation: 1) Simple cycloaddition of butane with ethylene (normal electron demand) giving rise to a single product; 2) Cycloaddition of 1,3-hexadiene and 1,3-dioxole (inverse electron demand, thermodynamic vs kinetic product formation) and finally 3) Possible reactions of sulphur dioxide with o-xylylene. It this last case, Diels-Alder cycloaddition could take place on either terminal cis-alkene or internal cis-alkene, giving rise to endo and exo products. On top of that, the two reactant can undergo a cheletropic reaction. The mutual degree of competitiveness between these five possible reaction outcomes will be investigated in exercise no. 3.
Methods
Based on the tutorials, method 3 was chosen to build molecules and compute their conformational minima and reaction transition states. For exercise number 1 and 3, semi-empirical method MP-6 was utilised, exercise number 2 was calculated on B3-LYP level.
Semi-empirical PM6 and B3LYP
Pm6 method uses several approximations and parametrised integrals which simplify the calculations making it a very fast way to compute the electronic states of molecule. Due to its fast calculation rate and reasonably accurate results, PM6 is commonly used to initially optimise molecules before moving to more complex and precise optimisation procedures. B3LYP is an exchange-correlation functional based on Hartree-Fock theory. B3LYP provides more accurate results, but the calculations take significantly longer time (i.e. are more expensive) than MP6.
TS defined correctly well done. Good understanding of PM6. Would have been good if you could have included HF and DFT.
Exercise 1: Cycloaddition of Butadiene with Ethylene
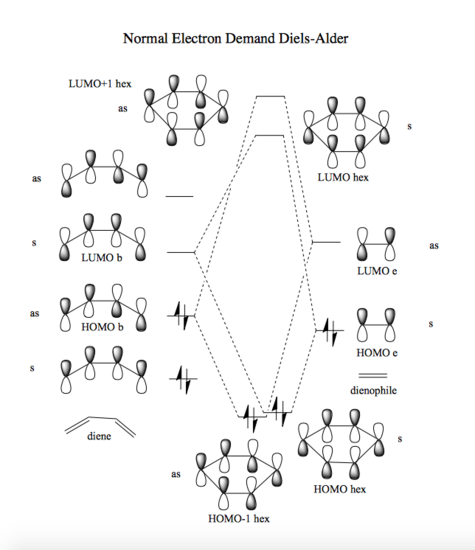
Picture no. 1 shows the energy pathway for this reaction and picture no. 2 illustrates in detail the participation of fragment molecular orbitals that contribute to the transition state. As a general rule, only those molecular orbitals of the same symmetry can combine, hence overlap integral of the symmetric + symmetric and asymmetric + asymmetric MO equals one, but overlap integral of asymmetric with symmetric or the other way around gives zero. [1] For a normal electron demand Diels-Alder reaction, the two occupied and unoccupied orbitals that are closer in energy and thus interact the most are HOMO of butadiene and LUMO of ethylene. Therefore the HOMO hex and "LUMO hex" of TS structure is a result of combination of ethylene HOMO and butadiene LUMO. (Note the difference with inverse electron demand DA in picture no. 3). Correct TS was confirmed by checking frequency and IRC calculation; one negative frequency was found and IRC curve looked as expected (i.e. products lower in E than reactants with TS being highest in E).
| Butadiene | Ethene | Transition State | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| |||||||||
|
|
|
The change in bond length along the reaction coordinate was visualised (see Graph no. 1). The standard single bond length is 1.54 Å [2] and standard double bond length 1.34 Å[2]. Given the Van der Waals radius of carbon atom is 1.70 Å [3], the minimal distance of two atoms being next to each other without sharing electron density is 2.4 Å. When looking at how the reaction progresses with time (Graph no. 1), the bond lengths between carbons C1C2 and C3C4 are 2.115 Å long at TS. This corresponds to a distance shorter than two times Van der Wals radius of interacting carbon nuclei, but this distance is much longer than a standard sp3 C-C bond. In other words, at the transition state, C1C2 and C3C4 are partially formed sigma bonds; the nuclei are close enough to interact and form a proper bond, but at the same time they are too far apart to be considered as a full single bond. Throughout the reaction C4C5 and C1C6 bonds elongate to 1.5 Å which is in a good agreement with a single bond dimension. C5C6 shortens to 1.3 Å, a diameter corresponding to a double bond and C2C3 distance prolongates to 1.54 Å as the double bond is being converted into a single bond. The animation of vibration at the TS suggests that the cycloaddition is a synchronous process i.e. the formation of product proceeds via concerted addition, which results in preservation of stereochemistry in products.

Transition State Vibration |
| Bond | Start | Transition State | End |
|---|---|---|---|
| C2C3 | 1.332 Å | 1.382 Å | 1.541 Å |
| C5C6 | 1.473 Å | 1.441 Å | 1.338 Å |
| C1C2, C3C4 | infinity | 2.115 Å | 1.540 Å |
| C1C6, C4C5 | 1.335 Å | 1.380 Å | 1.501 Å |
| Table 1: Change in bond length through reaction |
Nf710 (talk) 16:02, 10 February 2017 (UTC) Excellent first section. everything done well and conicisely
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole


In this exercise, the starting materials and TSs were optimised at B3LYP/6-31(G)d level.
In this case, two possible products were predicted to form: endo and exo, resulting from a different orientation of fragments on the reaction trajectory. The oxygens present on the dienophile act as electron donating groups, raising the dienophile HOMO and LUMO energy and as a consequence the two MOs closest in energy are HOMO of dioxole and LUMO of hexadiene. Hence HOMO and LUMO of TS is created from diene HOMO and dienophile LUMO (contrary to the previously discussed normal electron demand Diels-Alder MOs diagram (picture no. 1)) which is an evidence for Diels-Alder cycloaddition with an inverse electron demand. Frequency calculation was carried out for each stating material to assure that no negative frequency was present. The appropriate HOMO and LUMO at the TS were located (see the table at the end of this section) and matched with the Inverse Electron Demand Diels-Alder scheme (picture no. 3). The same rule was followed as for normal electron demand Diels-Alder: only orbitals of the same symmetry combined.
With reference to the scheme in picture no.4, both endo and exo product are very close in energy with a tiny difference of 3.60 kJ/mol. The exo product is kinetically favoured as it proceeds via transition state of lower energy (by only 7.84 kJ/mol) and hence it forms faster. On the other hand the endo product lies lower in energy which makes it thermodynamically more stable and favoured. The activation energies and reaction energies of the two possible products are so close in value that a fast equilibrium between the two products would be expected resulting in a final prevalence of the endo product.
| Starting Materials MOs | TS MOs |
|---|---|
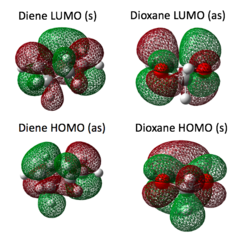 |
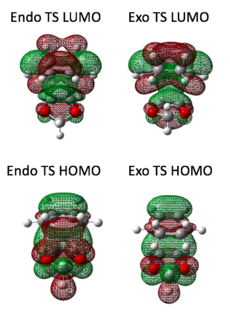 |
This is frustrating because, your energies are correct but you have got mixed up in your conclusions and not backed up your conclusions with arguments about SOO and sterics.
Exercise 3: Diels-Alder versus Cheletropic: Competing Reactions of Sulphur Dioxide and o-Xylylene
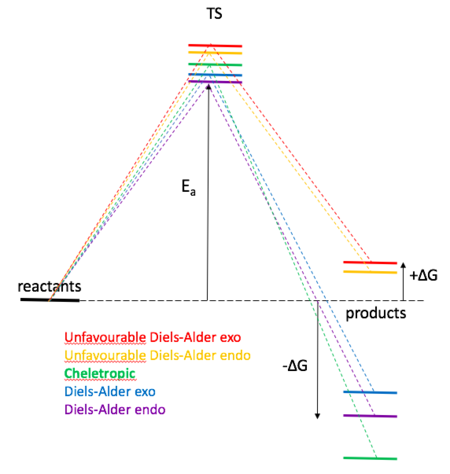
For the purpose of this exercise, the starting materials and TSs were optimised at PM6 level. Reaction of sulphur dioxide and o-xylylene can possibly result in 5 different products (picture no. 7); When reacting with teriminal cis-diene DA endo and DA exo product could be formed. Reaction at terminal diene yields an aromatic product which is favoured over reaction at the internal diene leading to non-aromatic product. The high instability of xylylene correlates to xylylene's high potential of being transformed into an aromatic product (hence become more stable). This transformation is clearly visible in the IRCs of terminal (favoured) Diels-Alder products as well as in cheletropic product IRC presented at the end of this section. Picture no. 8 is an illustration based on table no. 2 which summarises the energies of all species involved in possible transformations. The two highest TS energies were calculated for internal Diels-Alder cycloadditions. Other evidence of the internal cycloaddition reactions being highly unfavourable, provides the reaction energies, which are both positive. Positive ∆ G means that the reaction is not spontaneous in the stated direction. The product will not form under the given conditions. All products that arose from reacting of a terminal diene and sulphur are thermodynamically favourable, i.e. lower in E than starting material, probably due to the gain of aromaticity. Cheletropic product is the thermodynamic product, as it lies the lowest in energy and its TS is higher in E than terminal-alekene Diel-Alder products. The kinetic product would be then terminal Diels-Alder exo product. The products of internal cis-alkene Diels-Alder cycloaddition are kinetically unfavourable because their TS lies very high in energy (compared to cheletropic TS or terminal alkene Diels-Alder reaction) and also thermodynamically unfavourable because the products lie higher in energy than the starting material, hence their formation is highly unlikely.
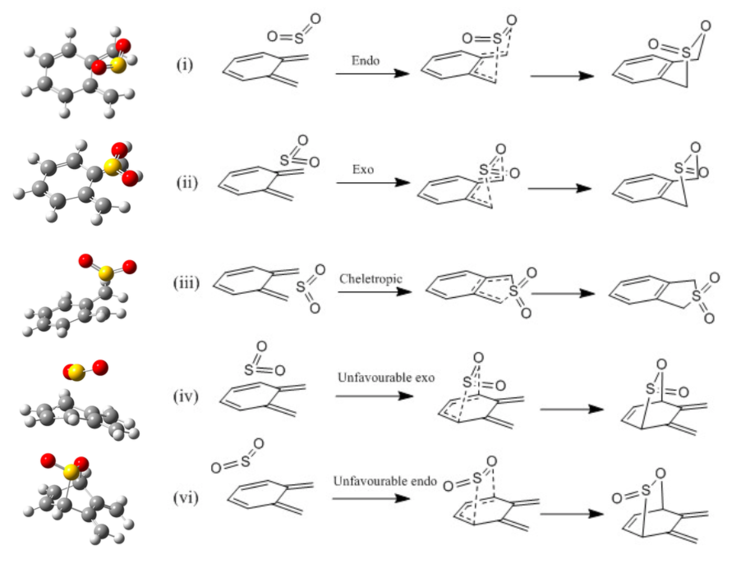

(Your endo product energy seems a bit off... perhaps not fully minimised Tam10 (talk) 14:34, 8 February 2017 (UTC))
Conclusion
Computational chemistry is a valuable tool to predict the reaction outcome based on energies of transition states. In this experiment, a normal electron demand Diels-Alder and reverse electron demand Diels-Alder reactions were investigated. In the first case, the HOMO of TS formed was from combination of dienophile HOMO and diene LUMO. In the later one, HOMO of diene and LUMO of dienophile combines to give TS HOMO. Diels-Alder Reactions might be competed with other types of reaction such as cheletropic reaction as seen in exercise 3. Both primary and secondary orbital interactions as well as additional energy contributions (aromatic gain/loss at TS, resonance, sterics...) have to be considered to fully account for the observed preference in product formation.
References
Template loop detected: Template:Reflist
- ↑ Template:D. Truhlar and B. garret and S. Klippenstein, "The Journal of Physical Chemisty", '''1996''', 1000 (31), 12771-12800
- ↑ 2.0 2.1 Template:H. Feilchenfeld, "The Journal of Physical Chemisty", '''1959''', 63 (8), 1346
- ↑ S. Batsanov, "Inorganic Materials Translated from Neorganicheskie Materialy Original Russian Text", 2001, 37, 871-885