Rep:Mod:ts wjb115
Introduction
Computational chemistry can provide a reliable and relatively quick method of understanding the mechanism and energetics of a reaction. It is particularly useful as it can be used to investigate reactions which cannot be done easily in the lab due to safety, cost or experimental difficulty.
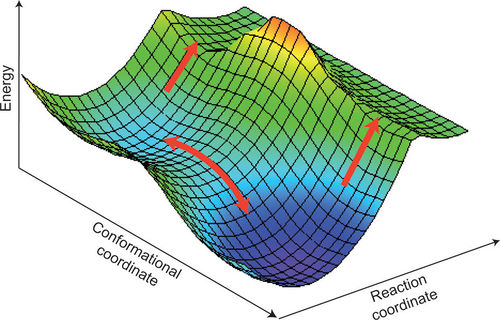
A potential energy surface is a way of expressing the potential energy of system, which in the case of this experiment involves the reactant and product species as well as the transition state structure. The PES can be used to analyse the molecular orientations and chemical reaction dynamics.
The minimum on a PES is a point at the which the gradient is equal to zero and the second derivative is positive, as there is positive curvature associated with a minimum and 3N-6 degrees of freedom. A distinction should also be made between local and global minima, whereby a global minimum is the absolute lowest point on a PES and a local minimum is a point which matches the criteria mentioned above.
On a PES a transition state corresponds to a saddle point, where the gradient is zero and the second derivative is negative along the reaction coordinate.
In order to obtain an optimized version of the transition state structure, knowledge of either the transition state, reactant approach orientation or products is necessary. As this is the case, three different methods were used during the experiment in order to find an optimized transition state structure.
Nf710 (talk) 17:16, 9 February 2018 (UTC) You need to be careful here and make sure you are talking in the degrees of freedom of the molecule. You get the force constants by diagonalising the hessian and getting its eigenvalues.
Method 1: A 'guess' transition transition state structure is drawn, followed by a transtion state optimisation
Method 2: A guess transition state structure is generated and then the reacting atoms are frozen. From here method 1 can be employed.
Method 3: Either the reactants or products are drawn. The bonds which will change during the reaction are altered and optimization is then done to a transition state.
Computational Methods
The Semi-empirical method is based on the Hartree-Fock formalism, but the method uses approximations and is parametized. Only valence electrons are considered during calculations, with effects from any core electrons being ignored.
When optimizing with the semi-empirical PM6 method certain one- and two-electron integrals from the Hamiltonian are ignored, and subsequently empirical parameters are included in the calculations. PM6 is based upon Hartree-Fock, in which a linear combination of atomic orbitals is used a basis set and a way of representing electronic wavefunctions. Using PM6 the number of integrals which need to be evaluated is greatly reduced and the time taken to run an optimisation decreases. The downside of this is that the accuracy of the optimization is also reduced, however, the time saved often makes the method useful.
The other method of optimisation used was the hybrid functional B3LYP/6-31G(d), which is based on DFT. The corresponding Hamiltonian for the B3LYP method is a function of charge density and below is an equation showing the exchange correlation part of the Hamiltonian. In order to account for this part of the Hamiltonian, which cannot be solved using DFT alone, B3LYP must use a Hartree-Fock method here. Consequently, this is a now a hybrid functional.
Nf710 (talk) 17:19, 9 February 2018 (UTC) Nice understanding of the QM methods. you have clearly done some extra reading here.
Exercise 1
MO Analysis

The MO diagram was produced by using a PM6 optimised structures of butadiene, ethene and the transition state. Since the transition state corresponds to the highest point on an energy profile, its MOs are subsequently higher than any of the frontier orbitals which are used to create the respective the MO.
Bonding and antibonding interactions are only observed between orbioatls of the same symmetry. For example symmetric and symmetric but not symmetric and antisymmetric.
The diagram displays mixing between the antisymmetric (AS) HOMO of butadiene and the AS LUMO of ethene. The LUMO of butadiene, which is symmetric, can mix with the symmetric HOMO of ethene to also produce transition state MOs.
The HOMO and LUMO MOs are the result of the overlap of between the symmetric HOMO of butadiene and LUMO of ethene. The 'HOMO-1' and 'LUMO+1' of the transition state are the result of interaction between the HOMO of butadiene and LUMO of ethene, which is a case of normal electron demand.
The orbitals discussed can be seen below.
| Butadiene | Ethene | Transition State | ||||||
|---|---|---|---|---|---|---|---|---|
Bond Length Analysis
| C-C Bond | C-C Bond Lengths (Å) | ||
| Butadiene/Ethene | Transition State | Cyclohexene | |
|---|---|---|---|
| C1-C2 | 1.33530 | 1.37976 | 1.50034 |
| C2-C3 | 1.46835 | 1.41111 | 1.33766 |
| C3-C4 | 1.33530 | 1.37976 | 1.50034 |
| C4-C5 | - | 2.11464 | 1.54040 |
| C5-C6 | 1.32754 | 1.38176 | 1.54076 |
| C6-C1 | - | 2.11477 | 1.54003 |
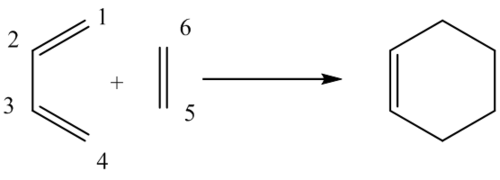
The numbered carbons in the reaction scheme, above, correspond to those in the table. For example C1-C2 is the bond between carbon 1 and carbon 2.
The length of the double bonds in butadiene (C1-C2, C3-C4)and ethene (C4-C5) increase in length during the reaction, and are longer in the transition state structure than initially in the reactants. In the product, cyclohexene, the C1-C2 and C3-C4 bonds are longer than in butadiene. This is because the hybridisation changes from sp2 to sp3. Similarly, the C5-C6 bond length increases in the transition state structure, and is also longer in cyclohexene than in ethene as the hybridisation changes from sp2 to sp3.
Conversely, the C2-C3 bond length decreases during the course of the reaction. This was expected since a single bond is converting into a double bond. The bond length in the transition state structure is longer than in cyclohexene as it does not have full double character until the product is fully formed. Here the hybridisation changes from sp3 to sp2.
It is reasonable to suggest that there is bonding in the transition state structure between C4-C5 and C1-C6. The bond length is roughly 2.11 Å, which is far shorter than twice the Van der Waals radius of 1.7 Å implying there is interaction between the electron densities of the two carbons and consequently a partially formed bond.
Literature values state typical sp3 C-C bond lengths as 1.54 Å and sp2 C=C bond lengths as 1.34 Å. When compared to the values obtained and displayed in the table above they are very similar. The length of the single bond in butadiene appears to be slightly shorter than literature would suggest, which could be a consequence of the optimisation underestimating the effect of conjugation in the butadiene molecule. As a result one would expect the single bonds to appear slightly shorter and the double bonds longer. The same could be said as to why the bond lengths of C3-C4 and C1-C2 are shorter than one would predict.
Vibrational Modes
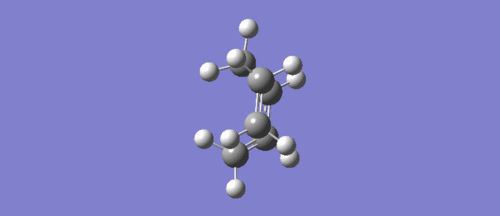
The above animation shows the imaginary vibration produced during the optimisation of the transisiton state structure. The terminal carbons of both reactants can be seen moving towards each other. Symmetry is maintained in this example, which one would expect in a Diels-Alder reaction.
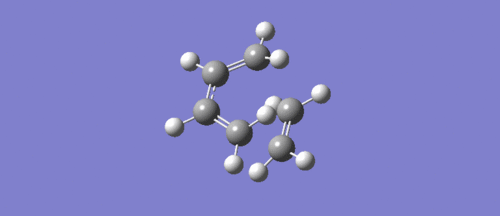
This is first real vibrational frequency, from the same transition state optimisation. The bonds do not move any closer to one another but instead just twist, so this frequency has nothing to do with bond formation.
(Fv611 (talk) It is very difficult to see whether this is the right vibration or not. I can't tell from this or from the Jmol inserted early on whether you have reached the correct transition state, which seems unlikely because the energy orderings in your MO diagram are off. You also forgot to answer both the question on orbital overlap and that on the synchronicity of bond forming.)
Exercise 2
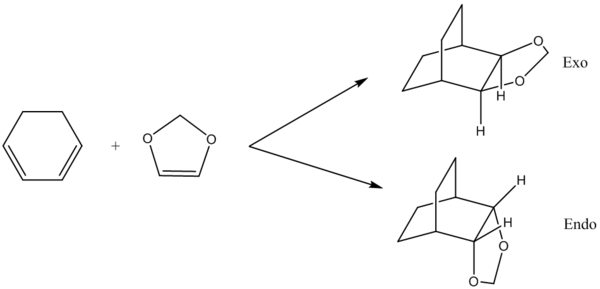
The reaction undertaken in this exercise was the Diels-Alder [4+2]-cycloaddition betweene cyclohexadiene and 1,3-dioxole. The reaction can produce two adducts, either endo or exo, depending on the orientation of the reacting molecules. Optimisation was done to the B3LYP level during this exercise.
MO analysis
(Fv611 (talk) Your MO diagram is good, but you do not mention or discuss at all the difference between the MO diagrams of the endo and exo reactions.)

The electron donating nature of the oxygen atoms adjacent to the double bond resulted in a high energy HOMO and LUMO in the dienophile. As a result of this the reaction proceeds via an inverse electron demand Diels-Alder. This is a consequence of the dioxole HOMO and cylcohexadiene LUMO having a smaller energy gap compared to the dioxole LUMO and cyclohexadiene LUMO. The smaller energy gap will be preferable and better overlap can occur between the HOMO of the dioxole and LUMO of the cyclohexadiene.
Nf710 (talk) 17:27, 9 February 2018 (UTC) how do you know this you have only stated this, you have not proved it
| Diene - LUMO | Dienophile - HOMO | Exo Transition State | Endo Transition State | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Thermodynamics
| Reaction Adduct | Activation Energy (kJ/mol) | Reaction Energy (kJ/mol) |
|---|---|---|
| Exo | 165.63 | -65.76 |
| Endo | 157.80 | -69.37 |
The above table shows reaction energy and activation of both the exo and endo reaction pathways. Using the activation energies one can conclude that the endo pathway of reaction is more kinetically favourable since the energy required to reach the transition state is lower. The lower, more negative reaction energy for the endo also suggests that it is the thermodynamically favoured pathway of reaction.
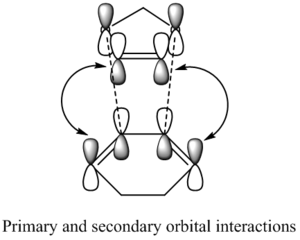
The reason as to why the endo has a lower activation energy compared to the exo pathway is because of secondary orbital interactions. The secondary orbital interactions can stabilise the endo transition state but not the exo. During the endo reaction a lone pair of electrons, located in a p orbital on the sp2 oxygen, is in the correct orientation to overlap with the orbitals on the diene and subsequent stanbilisation of the transition state occurs. This interaction is not possible for the exo pathway of reaction since the oxygen atoms are too great a distance from the diene. This is an explanation as to why the exo has a high activation energy than the endo.
Nf710 (talk) 17:31, 9 February 2018 (UTC) Your energies are pretty much correct. How have come to the correct conclusions however you could have gone into a bit more detail furthermore you could have quantitively proven if it was inverse or normal.
Exercise 3
 |
 |
 |
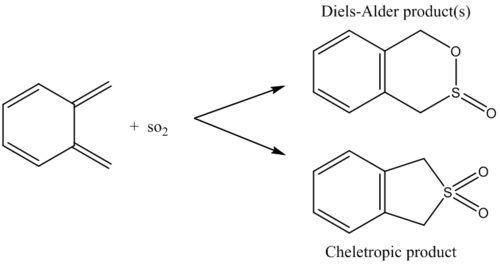
In the above animations one can see that the exo and endo reactions are both asynchronous whilst the cheletropic is synchronous.
The reason for the instability of xylylene, in all three reaction pathways, is due to the 6-membered ring becoming delocalised thus meaning there is a strong driving force for aromatisation.
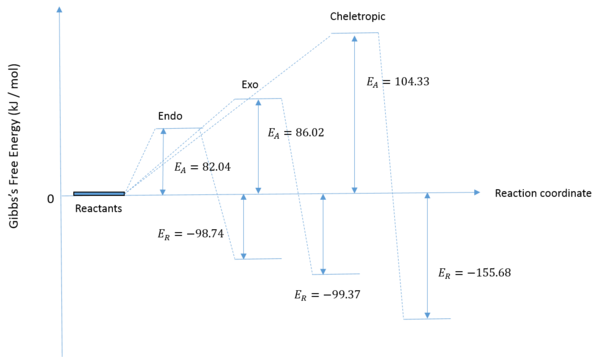
Thermochemistry
| Reaction Adduct | Activation Energy (kJ/mol) | Reaction Energy (kJ/mol) |
|---|---|---|
| Endo Diels-Alder | 82.04175 | -98.73 |
| Exo Diels-Alder | 86.016 | -99.372 |
| Cheletropic | 104.325375 | -155.680875 |
The data obtained and displayed in the above table shows that the exo adduct is the thermodynamic product whilst the endo adduct is the kinetic product. In the reaction profile provided the relative activation energies of the three reactions were plotted, as well as the relative reaction energies. It can be seen that the Cheletropic reaction has the largest activation energy and leads to the formation of the most stable product. The high activation energy is a consequence of the formation of a strained five-membered ring. The strain of the ring is compensated by the strong S=O bond which increases the stability of the product to such an extent that it is the thermodynamic product.
Alternative reaction pathway with other cis-butadiene fragment
| Reaction Adduct | Activation Energy (kJ/mol) | Reaction Energy (kJ/mol) |
|---|---|---|
| Endo Diels-Alder | 112.24 | 16.53 |
| Exo Diels-Alder | 120.07 | 20.98 |
Since there is another cis-diene present in the molecule an alternative reaction should be possible. As shown in the results above, both exo and endo pathways of this reaction have higher activation energies. this makes these pathways very kinetically unfavourable. Not only this but having positive reaction energies implies endothermic reactions and thus energy must be put in to make them happen, so they are also thermodynamically unfavourable as well as kinetically. A loss of aromaticity is responsible for this.
 |
 |
Electrocyclic ring closing: A Butadiene
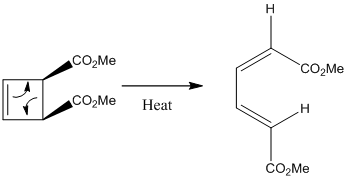
Using Woodward-Hoffmann rules one can determine that this reaction would proceed in a conrotatory fashion, since it is π^4 system. Thus, the under thermal conditions it must proceed via conrotation and not disrotation.

(Well done finding this TS. You could have done some more analysis on the MOs and predicted what would happen on the electronically excited state Tam10 (talk) 14:49, 4 February 2018 (UTC))
(+4%)
Conclusion
Using the methods PM6 and B3LYP allowed for the calculation of reaction and activation energies and subsequently the feasibilty of the Diels-Alder reactions could be assessed.
The PM6 method was by far the quicker of the two methods and meant a TS structure could be reached quickly. However, if accurate results are required then B3LYP should certainly be used instead.
Exercise 1 looked at a Diels-Alder, with normal electron demand, and MO analysis was done. The bond lengths obtained during the exercise were similar to those in literature. Exercise 2 looked at an inverse electron demand Diels-Alder, and the thermochemistry was considered. The outcome of the thermodynamic calculations showed that the endo adduct was both the kinetic and thermodynamic product. In the final exercise a cheletropic reaction was shown to be the most thermodynamically stable despite its much larger activation energy, due the strain of the 5-membered ring.
References
1. David R. Glowacki, Jeremy N. Harvey & Adrian J. Mulholland, Nature Chemistry volume 4, pages 169–176 (2012)
2. J. Clayden, N. Greeves and S. Warren, Organic Chemistry, 2nd edition, 2001
3. J. W. McDouall, Computational Quantum Chemistry: Molecular Structure and properties, 2013
