Rep:Mod:ts cyy113 edit
| Species or IRC | Basis Set |
|---|---|
| PM6 | |
| o-xylylene | File:Xylene cyy113.log |
| SO2 | File:SO2 CYY113.LOG |
| Endo TS | File:EX3ENDOTS CYY113.LOG |
| Exo TS | File:XYLYLENE exoex3 TS cyy113.LOG |
| Cheletropic TS | File:INDENE TS cyy113.LOG |
| Endo Product | File:ENDOPRODUCT cyy113ex3.LOG |
| Exo Product | File:EXOPRODUCT OPT CYY113.LOG |
| Cheletropic Product | File:INDENEPRODUCT CYY113.LOG |
| Endo IRC | File:EX3ENDOTSIRC cyy113.LOG |
| Exo IRC | File:Xylene exo ts irccyy113.log |
| Cheletropic IRC | File:INDENEIRC CYY113.LOG |
| Xylylene | SO2 | Endo TS | |||
|---|---|---|---|---|---|
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.178746 | 469.297659 | -0.118614 | -311.4210807 | 0.102070 | 267.98480541 |
| Endo Product | Exo TS | Exo Product | |||
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.065609 | 172.25644262 | 0.105054 | 275.81929801 | 0.067306 | 176.711916 |
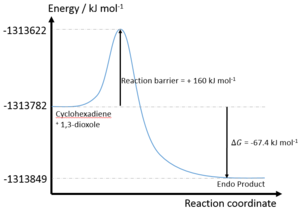
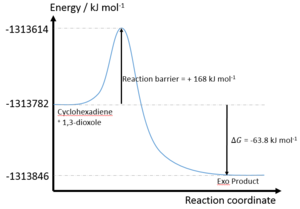
| Reaction | Formation of Endo Product | Formation of Exo Product |
|---|---|---|
| Reaction barrier/ kJ mol-1 | +110.1 | +117.9 |
| Reaction energy/ kJ mol-1 | +14.4 | +18.8 |
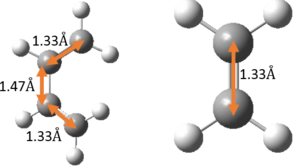
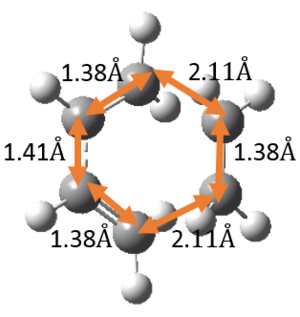
The relevant C-C bond lengths are tabulated below:
| Reactant C-C bond lengths | TS C-C bond lengths | Product C-C bond lengths |
|---|---|---|
|
|

|
| Reported sp2 and sp3 bond lengths
in cyclohexene[1] /Å |
Typical sp2 and
sp3 bond lengths[2] /Å |
Van Der Waals' radius
of C atom[3] /Å |

|
sp2 = 1.31-1.34
sp3 = 1.53-1.55 |
0.77 |
Write whether the orbital overlap integral is zero or non-zero for the case of a symmetric-antisymmetric interaction, a symmetric-symmetric interaction and an antisymmetric-antisymmetric interaction.
The orbital overlap integral quantifies the extent of overlap between reactant MOs (denoted by and . It is given by the following equation.[4]
The possible values of depends on and and they are as follows:
| Type of interaction | Symmetric-Antisymmetric | Symmetric-Symmetric | Antisymmetric-Antisymmetric |
|---|---|---|---|
| Value of S |
intro
In your introduction, briefly describe what is meant by a minimum and transition state in the context of a potential energy surface. What is the gradient and the curvature at each of these points? (for thought later on, how would a frequency calculation confirm a structure is at either of these points?)
The dynamics of a chemical reaction can be investigated using a potential energy surface (PES) obtained by plotting against and , where is the potential energy and , are order parameters of a reaction. Reactants and products occur at the minimum of the PES while transition states occur at the saddle point. Mathematically, they are both stationary points, so the gradient (i.e. the first derivative ) of both the minimum and the saddle point is 0. However, the curvature of the minimum is concave while that of the saddle point is convex. Thus, they can only be distinguished by taking the second derivative , which will be positive for the minimum, but negative for the saddle point.
Since a chemical bond can be modelled as a spring, Hooke's Law (eq. 1) can be invoked, where is the elastic potential energy, is a constant and is the displacement of the particle from its equilibrium position.
Taking the second derivative of this equation results in eq. 2. Thus, will be positive for all minimum points but negative for saddle points. Physically, this means that all coordinates have been minimised for reactants and products, but some coordinates have not been minimised for transition states. Thus, reactants and products remain stable unless they are excited with energy but transition states are inherently unstable and will rearrange into a stable form spontaneously.
Since the vibrational frequency is a function of (eq. 3), reactants and products will always give real frequencies, while transition states will give imaginary frequencies. A well-chosen reaction coordinate will only result in a transition state with one imaginary frequency, as all other coordinates have been minimised.
In these exercises, computational methods were used to determine suitable reaction coordinates of several [4+2] Diels-Alder cycloadditions. The coordinates of the reactants, transition state (TS) and products were optimised using Gaussian, from which an Intrinsic Reaction Coordinate (IRC) calculation was run to visualise the approach trajectory of the reactants to form a transition state and subsequently the product. Further observable parameters (Bond Length and Energies) were also analysed from the optimised coordinates and IRC calculation. The wavefunctions of the optimised species were visualised as molecular orbitals (MOs) and their shapes and symmetries were compared to theoretical predictions.
Optimisation was conducted at the semi-empirical PM6 level first, before some calculations were refined using Density Functional Theory (DFT) methods at the BY3LP/6-31(d) level. The PM6 optimisation uses a fitted method drawing on experimental data, hence it is less accurate. However, it saves computational resources and is a good starting point for initial calculations. The BY3LYP/6-31(d) optimisation is more accurate but it comes at the cost of computational effort.
MO Analysis
Using your MO diagram for the Diels-Alder reaction, locate the occupied and unoccupied orbitals associated with the DA reaction for both TSs by symmetry. Find the relevant MOs and add them to your wiki (at an appropriate angle to show symmetry). Construct a new MO diagram using these new orbitals, adjusting energy levels as necessary.
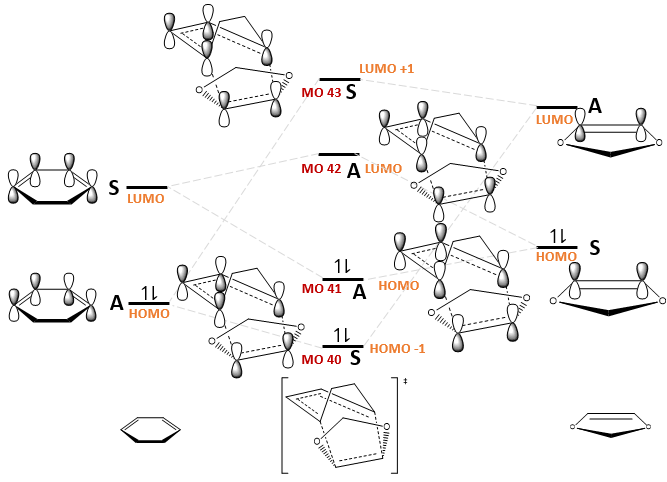
The MO diagrams for the formation of the endo and exo transition states, as well as the relevant MOs, are shown in Fig. 9 and Table 7 respectively. The relative ordering of the MO energies were based on the orbital energies obtained from a BY3LYP/6-31(d) optimisation of the reactants and transition states, but exact energies were not considered for simplicity.
 |
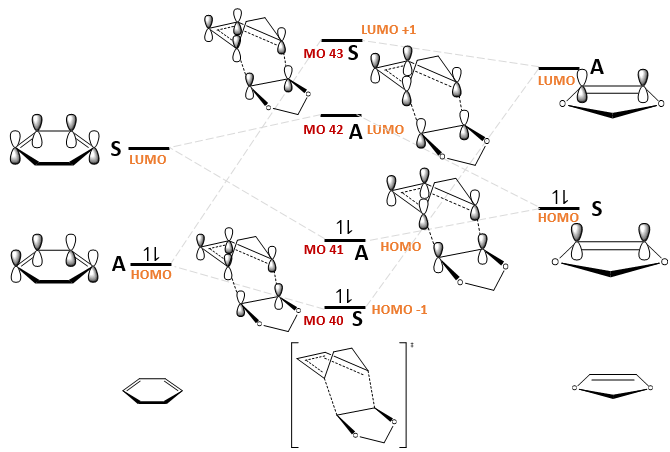 |
| Endo TS | Exo TS | |
|---|---|---|
| LUMO+1 (MO 43) | ||
| LUMO (MO 42) | ||
| HOMO (MO 41) | ||
| HOMO -1 (MO 40) |
Fig. 11 shows that secondary orbital interactions exist only for the endo TS. This has a stabilising effect as demonstrated in Fig. 12. Note that not all the orbitals are drawn; only those interacting are shown for simplicity.
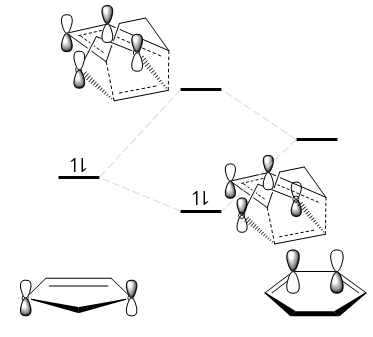
This secondary orbital interaction explains the endo rule, which states that the endo product is always preferentially formed in a Diels-Alder reaction.[5]
| Endo TS | Exo TS | Cheletropic |
|---|---|---|
| endo | exo | che |
| Xylylene | SO2 | Endo TS | Endo Product | ||||
|---|---|---|---|---|---|---|---|
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.178746 | 469.297659 | -0.118614 | -311.4210807 | 0.090562 | 237.770549 | 0.021701 | 56.9759798 |
| Exo TS | Exo Product | Cheletropic TS | Cheletropic Product | ||||
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.092078 | 241.750807 | 0.021453 | 56.3248558 | 0.099062 | 260.087301 | 0.000005 | 0.013127501 |
| Reaction | Formation of Endo Product | Formation of Exo Product | Formation of Cheletropic Product |
|---|---|---|---|
| Reaction barrier/ kJ mol-1 | +79.9 | +83.9 | +102.2 |
| Reaction energy/ kJ mol-1 | -100.9 | -101.6 | -157.9 |
- ↑ J. F. Chiang and S. H. Bauer, Journal of the American Chemical Society, 1969, 91, 1898–1901
- ↑ E. V. Anslyn and D. A. Dougherty, Modern physical organic chemistry, Univ. Science Books, Sausalito, CA, 2008, pp.22
- ↑ G. S. Manku, Theoretical principles of inorganic chemistry, Inter-India Publ., New Delhi, 1986, pp.97
- ↑ P. Atkins and J. De Paula, Atkins' Physical Chemistry, University Press, Oxford, 10th edn., 2014.
- ↑ J. García, J. Mayoral and L. Salvatella, European Journal of Organic Chemistry, 2004, 2005, 85-90.