Rep:Mod:ts cyy113
Introduction
In your introduction, briefly describe what is meant by a minimum and transition state in the context of a potential energy surface. What is the gradient and the curvature at each of these points? (for thought later on, how would a frequency calculation confirm a structure is at either of these points?)
The dynamics of a chemical reaction can be investigated using a potential energy surface (PES) obtained by plotting against and , where is the potential energy and , are order parameters of a reaction. Reactants and products occur at the minimum of the PES while transition states occur at the saddle point.[1] Mathematically, they are both stationary points, so the gradient (i.e. the first derivative ) of both the minimum and the saddle point is 0. However, the curvature of the minimum is concave while that of the saddle point is convex. Thus, they can only be distinguished by taking the second derivative , which will be positive for the minimum, but negative for the saddle point.
Nf710 (talk) 09:59, 16 November 2017 (UTC) Careful here. Your have defined your PES with only 2 dimensions. It is 3N-6 dimensions. secondly at a saddle point you need at least 2 dimensions so it is the curvature of only 1 that is convex at a first order saddle point.
Since a chemical bond can be modelled as a spring[1], Hooke's Law (eq. 1) can be invoked, where is the elastic potential energy, is a constant and is the displacement of the particle from its equilibrium position.
Taking the second derivative of this equation results in eq. 2. Thus, will be positive for all minimum points but negative for saddle points. Physically, this means that all coordinates have been minimised for reactants and products, but some coordinates have not been minimised for transition states. Thus, reactants and products remain stable unless they are excited with energy but transition states are inherently unstable and will rearrange into a stable form spontaneously.
Nf710 (talk) 11:11, 16 November 2017 (UTC) Again how many dimensions are you talking about
Since the vibrational frequency is a function of (eq. 3)[1], reactants and products will always give real frequencies, while transition states will give imaginary frequencies. A well-chosen reaction coordinate will only result in a transition state with one imaginary frequency, as all other coordinates have been minimised.
In these exercises, computational methods were used to determine suitable reaction coordinates of several [4+2] Diels-Alder cycloadditions. The coordinates of the reactants, transition state (TS) and products were optimised using Gaussian, from which an Intrinsic Reaction Coordinate (IRC) calculation was run to visualise the approach trajectory of the reactants to form a transition state and subsequently the product. Further observable parameters (Bond Length and Energies) were also analysed from the optimised coordinates and IRC calculation. The wavefunctions of the optimised species were visualised as molecular orbitals (MOs) and their shapes and symmetries were compared to theoretical predictions.
Optimisation was conducted at the semi-empirical PM6 level first, before some calculations were refined using Density Functional Theory (DFT) methods at the BY3LP/6-31(d) level. The PM6 optimisation uses a fitted method drawing on experimental data, hence it is less accurate. However, it saves computational resources and is a good starting point for initial calculations. The BY3LYP/6-31(d) optimisation is more accurate but it comes at the cost of computational effort.
Exercise 1
(Fv611 (talk) Very well done. A well structured answer and very good critical discussions across the whole exercise.)
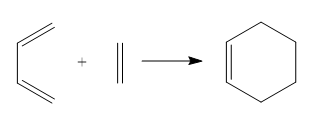
The following Diels-Alder cycloaddition was investigated. Butadiene was the diene while ethene was the dienophile. Butadiene had to be in the correct s-cis conformation so that there is effective spatial overlap with ethene. Reactants, TS and products were optimised at the PM6 level.
Optimisation Results
1) Optimise the reactants and TS at the PM6 level.
| Butadiene (s-cis) | Ethene | TS | ||||||
|---|---|---|---|---|---|---|---|---|
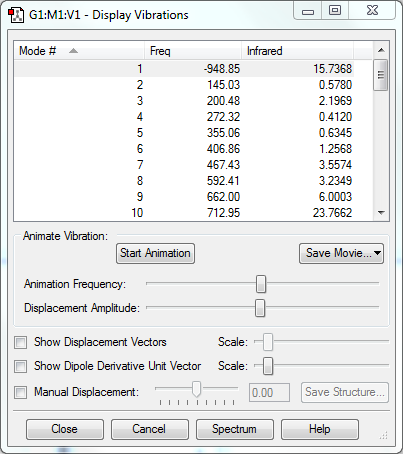
2) Confirm that you have the correct TS with a frequency calculation and IRC.
Frequency Calculation
IRC
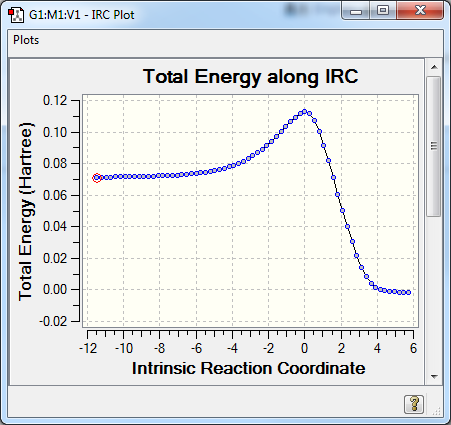
A well-defined, asymmetric Free Energy Surface was obtained, further confirming that the reaction coordinate was well chosen. (Fig. 4) A predicted trajectory was computed using a Intrinsic Reaction Coordinate function on Gaussian. (Fig. 5)
 |
 |
3) Optimise the products at the PM6 level.
| Cyclohexene | ||
|---|---|---|
Analysis of MO diagrams
Construct an MO diagram for the formation of the butadiene/ethene TS, including basic symmetry labels (symmetric/antisymmetric or s/a).
Based on the values obtained from a Gaussian calculation using the PM6 basis set, secondary orbital mixing between the MOs of the transition state is expected, which stabilises LUMO+1 and destabilises the HOMO, as illustrated with the red arrows and energy levels.
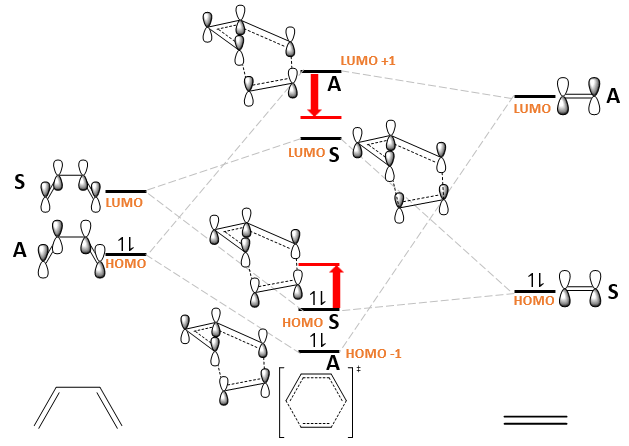
The HOMO-LUMO energy gap in butadiene is smaller than ethene due to increased conjugation. In fact, this reaction is reported to proceed inefficiently.[2] The reaction will proceed more efficiently if butadiene is made more electron rich by adding electron donating groups, or ethene is made more electron poor by adding electron withdrawing groups. Adding electron donating groups raises the energy levels, while adding electron withdrawing groups lower the energy levels. This effectively reduces the HOMO-LUMO energy gap allowing for more efficiency overlap.
(Fv611 (talk) Good discussion about the reaction efficiency.)
For each of the reactants and the TS, open the .chk (checkpoint) file. Under the Edit menu, choose MOs and visualise the MOs. Include images (or Jmol objects) for each of the HOMO and LUMO of butadiene and ethene, and the four MOs these produce for the TS. Correlate these MOs with the ones in your MO diagram to show which orbitals interact.
|
|
(Fv611 (talk) Would have been helpful to add symmetry labels to the MO table, or to number them both in the table and in the MO diagram.)
What can you conclude about the requirements for symmetry for a reaction (when is a reaction 'allowed' and when is it 'forbidden')?
The MOs of the transition state are all formed by overlapping and mixing reactant MOs of the same symmetry (Fig. 5), suggesting that a reaction is only allowed when reactant MOs of the same symmetry overlap. This can be justified mathematically. As the coordinates of the atoms change along a reaction coordinate, the wavefunctions of the reactant MOs (denoted as and ) can no longer describe the electron density of the transition state[3]. A transition state wavefunction (denoted as ) will better describe this electron density (as ). The energy of the transition state wavefunction is given via the Hamiltonian as follows:
The Hamiltonian is a totally symmetric operator, meaning that has the same symmetry as . If the symmetries of and were different, would be the sum of and , which cannot be the case since the reactants are not at infinite separation.
Write whether the orbital overlap integral is zero or non-zero for the case of a symmetric-antisymmetric interaction, a symmetric-symmetric interaction and an antisymmetric-antisymmetric interaction.
The orbital overlap integral quantifies the extent of overlap between reactant MOs (denoted by and ). It is given by the following equation.[3]
The possible values of depends on and and they are as follows:
| Type of interaction | Symmetric-Antisymmetric | Symmetric-Symmetric | Antisymmetric-Antisymmetric |
|---|---|---|---|
| Value of |
(Fv611 (talk) Very good symmetry discussion, although you could have pointed out that the orbital overlap is directly related to the symmetry requirements, as the reaction cannot happen with overlap zero.)
Bond Length Analysis
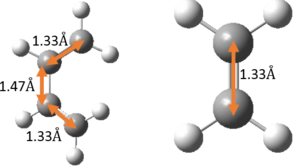
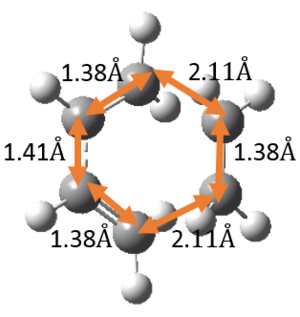
Include measurements of the 4 C-C bond lengths of the reactants and the 6 C-C bond lengths of the TS and products. How do the bond lengths change as the reaction progresses? What are typical sp3 and sp2 C-C bond lengths? What is the Van der Waals radius of the C atom? How does this compare with the length of the partly formed C-C bonds in the TS.
The relevant C-C bond lengths are tabulated below:
| Reactant C-C bond lengths | TS C-C bond lengths | Product C-C bond lengths |
|---|---|---|
|
|

|
| Reported sp2 and sp3 bond lengths
in cyclohexene[4] /Å |
Typical sp2 and
sp3 bond lengths[5] /Å |
Van Der Waals' radius
of C atom[6] /Å |

|
sp2-sp3 C-C = 1.50 |
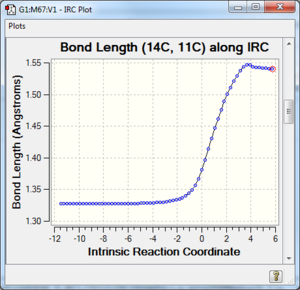
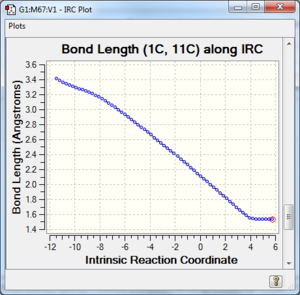
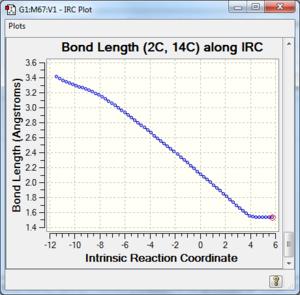
The partly formed C-C bond lengths are between a (C-C) single bond length and twice the Van Der Waals radius of C. This shows that a bond is indeed being formed. In addition, the C-C bond lengths in the cyclohexene product agreed well with literature values of cyclohexene and average sp2 C sp2 C, sp2 C sp3 C and sp3 C sp3 C bond lengths as well. This is further support that the cyclohexene product and TS has been optimised well.
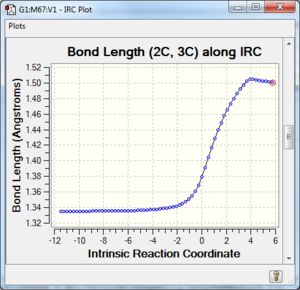
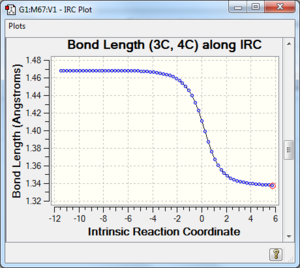
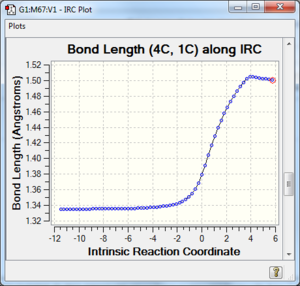
Along the reaction coordinate, graphs showing the change in various C-C bond lengths are shown in the table below. The atoms are labelled based on Fig. 8.
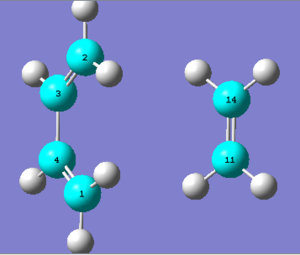
Vibrational Analysis
Illustrate the vibration that corresponds to the reaction path at the transition state. Is the formation of the two bonds synchronous or asynchronous?
The vibration that corresponds to the reaction path occurs at . The formation is synchronous as expected of a typical Diels Alder cycloaddition.
| Fig. 9: Reaction path vibration for Butadiene/Ethene TS |
(Fv611 (talk) Very thorough analysis. The reference to literature values for cyclohexene was a very good addition.)
Exercise 2
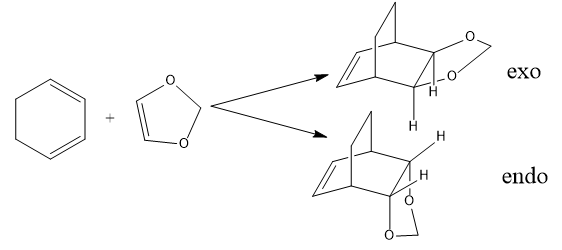
In this exercise, the following reaction was investigated. Cyclohexadiene was the diene while 1,3-dioxole was the dienophile. Reactants, TS and products were optimised at both the PM6 and BY3LYP/6-31(d) levels.
Optimisation Results
Using any of the methods in the tutorial, locate both the endo and exo TSs at the B3LYP/6-31G(d) level (Note that it is always fastest to optimise with PM6 first and then reoptimise with B3LYP).
Method 3 was used and the following optimisations were obtained:
| Endo TS | Exo TS | Cyclohexadiene | 1,3-dioxole | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM6 | ||||||||||||
| B3LYP/6-31G(d) |
Frequency Analysis
Confirm that you have a TS for each case using a frequency calculation.
The frequencies of each TS are shown in the table below. In each case, there is only 1 negative frequency which confirms that a TS has been located on a suitable reaction coordinate.
| Endo TS | Exo TS | |||
|---|---|---|---|---|
| Basis Set | PM6 | BY3LYP/6-31G(d) | PM6 | BY3LYP/6-31G(d) |
| Frequencies | 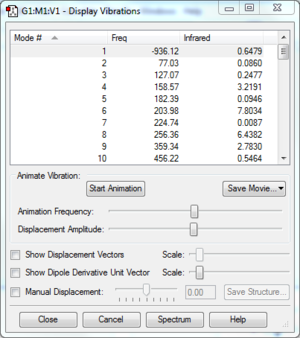 |
 |
 |
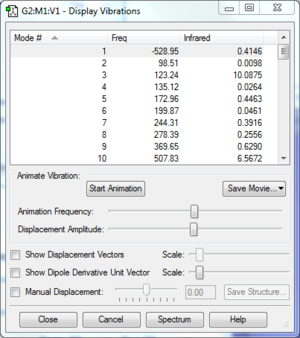 |
MO Analysis
Using your MO diagram for the Diels-Alder reaction, locate the occupied and unoccupied orbitals associated with the DA reaction for both TSs by symmetry. Find the relevant MOs and add them to your wiki (at an appropriate angle to show symmetry). Construct a new MO diagram using these new orbitals, adjusting energy levels as necessary.
The MO diagrams for the formation of the endo and exo transition states, as well as the relevant MOs, are shown in Fig. 11 and Table 7 respectively. The relative ordering of the MO energies were based on the orbital energies obtained from a BY3LYP/6-31(d) optimisation of the reactants and transition states, but exact energies were not considered for simplicity.
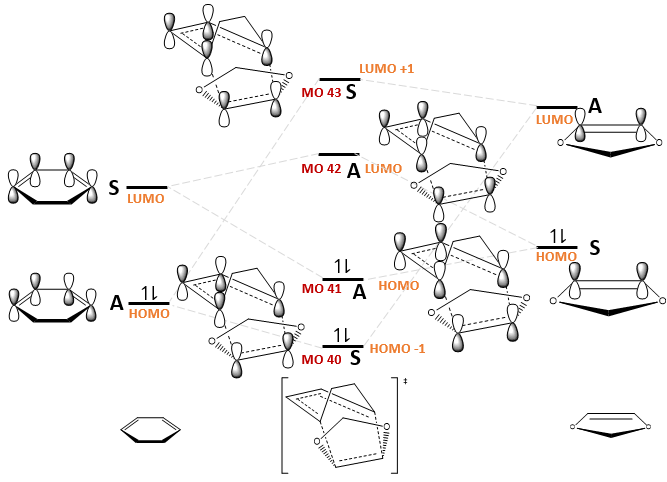 |
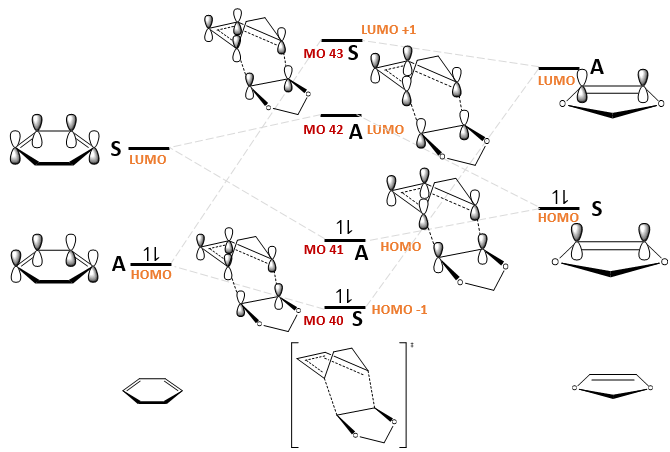 |
(Why are the symmetry labels switching for your TS MOs? Tam10 (talk) 13:34, 10 November 2017 (UTC))
| Endo TS | Exo TS | |||||
|---|---|---|---|---|---|---|
| LUMO+1 (MO 43) | ||||||
| LUMO (MO 42) | ||||||
| HOMO (MO 41) | ||||||
| HOMO -1 (MO 40) |
Is this a normal or inverse demand DA reaction?
This is an inverse demand DA reaction. In a normal demand DA reaction, the HOMO of the diene reacts with the LUMO of the dienophile but in an inverse demand DA reaction, the HOMO of the dienophile reacts with the LUMO of the diene.[7] Thus, in this reaction, the HOMO of the dienophile 1,3-dioxole reacted with the LUMO of the diene, hexadiene. This can be rationalised by Molecular Orbital (FMO) theory, which states that the rate of a Diels-Alder reaction is faster if the energy gap between the overlapping HOMO and LUMO orbitals is smaller.[7] Based on the energy calculations of cyclohexadiene and 1,3-dioxole at the BY3LYP/6-31(d) level, it is found that the energy gap between LUMO (hexadiene) and HOMO (1,3-dioxole) is smaller than that between HOMO (hexadiene) and LUMO (1,3-dioxole).
In addition, the symmetries of the LUMO +1, LUMO, HOMO and HOMO +1 molecular orbitals in the transition state are all reversed in an inverse demand Diels-Alder reaction. This is reflected in the A-S-S-A ordering of these transition state MOs, which should have been S-A-A-S in a normal demand Diels-Alder reaction.
In fact, it is expected that this Diels Alder cycloaddition proceeds with inverse demand. Electrons can be donated into the dienophilic double bond via resonance effect from the adjacent oxygen atoms. Thus, the energies of the dienophile MOs will increase in energy[7] such that the HOMO of the dienophile will be closer in energy to the LUMO of the diene.
Reaction Barriers and Reaction Energies
In the .log files for each calculation, find a section named "Thermochemistry". Tabulate the energies and determine the reaction barriers and reaction energies (in kJ/mol) at room temperature (the corrected energies are labelled "Sum of electronic and thermal Free Energies", corresponding to the Gibbs free energy).
The energies of reactants, TS and products are summarised in the table below.
| Cyclohexadiene | 1,3-dioxole | Endo TS | |||||
|---|---|---|---|---|---|---|---|
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | ||
| Basis Set | PM6 | 0.116877 | 306.860587 | 0.052276 | 137.250648 | 0.137939 | 362.158872 |
| BY3LYP/6-31(d) | -233.324375 | -612593.19323 | -267.068650 | -701188.79399 | -500.332148 | -1313622.1546 | |
| Exo TS | Endo Product | Exo Product | |||||
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | ||
| Basis Set | PM6 | 0.138903 | 364.689854 | 0.037804 | 99.2544096 | 0.037977 | 99.7086211 |
| BY3LYP/6-31(d) | -500.329168 | -1313614.3306 | -500.418691 | -1313849.3733 | -500.417320 | -1313845.77374 | |
The reaction barriers (i.e. activation energy) and reaction energies (i.e. ) were then calculated using energy values obtained from the BY3LYP/6-31G(d) basis set, as they are more accurate. The reaction barrier is the difference between the energies of the TS and the reactants, while the reaction energy is the difference between the energies of the reactants and products. They are summarised in the table below:
| Reaction | Formation of Endo Product | Formation of Exo Product |
|---|---|---|
| Reaction barrier/ kJ mol-1 | +160 | +168 |
| Reaction energy/ kJ mol-1 | -67.4 | -63.8 |
Which are the kinetically and thermodynamically favourable products?
In this reaction, the endo product has a lower activation energy and hence the kinetically favourable product. This is in agreement with the fact that secondary orbital interactions exist only for the endo product in any Diels-Alder cycloaddition involving substituted dienophiles.[8] In this example, it is illustrated in Fig. 13.
In addition, in this reaction the endo product has a more negative reaction energy and is hence the thermodynamically favourable product. This deviates from the fact that the endo product suffers from steric clash. However, since a significant extent of distortion is required from cyclohexadiene to form the transition state[9], it is likely that the reaction proceeds with a late transition state. By Hammond's Postulate, this means that the transition state resembles the products[10]. As such, the same secondary orbital interactions that stabilise the endo transition state will also stabilise the endo product, leading to a more negative reaction energy.
Nf710 (talk) 11:37, 16 November 2017 (UTC) This is a good theory with Hammonds postulate. You can actually see this in the MOs of the product. Howevrr in this caze the sterics are only present in the exo
Look at the HOMO of the TSs. Are there any secondary orbital interactions or sterics that might affect the reaction barrier energy (Hint: in GaussView, set the isovalue to 0.01. In Jmol, change the mo cutoff to 0.01)?
| HOMO of Endo TS | HOMO of Exo TS | ||||
|---|---|---|---|---|---|
Fig. 12 shows that secondary orbital interactions exist only for the endo TS. This has a stabilising effect as demonstrated in Fig. 13. Note that not all the orbitals are drawn; only those interacting are shown for simplicity.
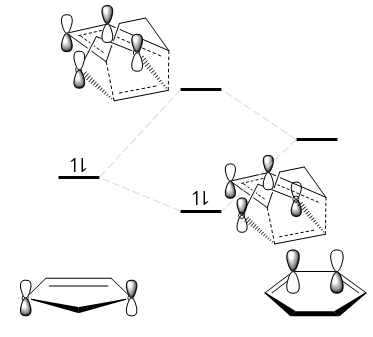
This secondary orbital interaction explains the endo rule, which states that the endo product is always preferentially formed in a Diels-Alder reaction.[8]
Exercise 3
The Diels-Alder reaction between o-xylylene and SO2 is investigated in this exercise. o-xylylene is the diene while SO2 is the dienophile.
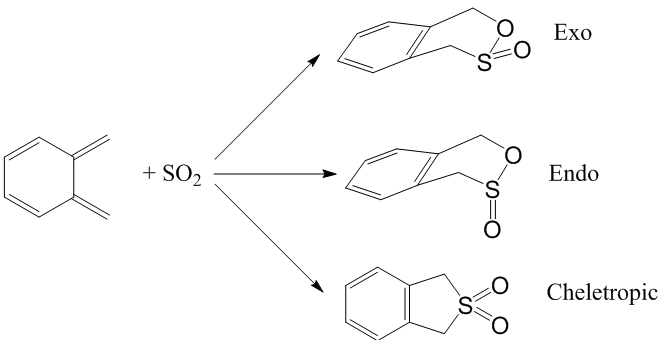
Optimisation Results
1) Optimise the TSs for the endo- and exo- Diels-Alder and the Cheletropic reactions at the PM6 level.
The endo and exo TS each have a pair of enantiomers. In each case, only 1 enantiomer is displayed in the JMol files in the figure below.
| Exo TS | Endo TS | Cheletropic TS | ||||||
|---|---|---|---|---|---|---|---|---|
(Make sure you use the correct frame to display your Jmols. These are all showing the input file Tam10 (talk) 13:38, 10 November 2017 (UTC))
Reaction Barriers and Reaction Energies
3) Calculate the activation and reaction energies (converting to kJ/mol) for each step as in Exercise 2 to determine which route is preferred.
The energies of the reactants, TS and products for the endo, exo and cheletropic reactions are summarised below:
| o-xylylene | SO2 | Endo TS | Endo Product | ||||
|---|---|---|---|---|---|---|---|
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.178044 | 467.454558 | -0.118614 | -311.4210807 | 0.090562 | 237.770549 | 0.021701 | 56.9759798 |
| Exo TS | Exo Product | Cheletropic TS | Cheletropic Product | ||||
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.092078 | 241.750807 | 0.021453 | 56.3248558 | 0.099062 | 260.087301 | 0.000005 | 0.013127501 |
Taking the same differences in energies as in Exercise 2, the reaction barrier (activation energy) and reaction energies are as follows:
| Reaction | Formation of Endo Product | Formation of Exo Product | Formation of Cheletropic Product |
|---|---|---|---|
| Reaction barrier/ kJ mol-1 | +81.7 | +85.7 | +104.0 |
| Reaction energy/ kJ mol-1 | -99.1 | -99.4 | -155.7 |
4) Using Excel or Chemdraw, draw a reaction profile that contains relative heights of the energy levels of the reactants, TSs and products from the endo- and exo- Diels-Alder reactions and the cheletropic reaction. You can set the 0 energy level to the reactants at infinite separation.
The relative energy levels are shown in the figure below, generated on ChemDraw.
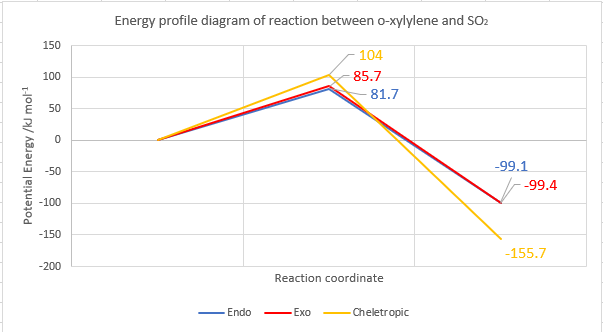
Between the endo/exo pathways, the endo product is kinetically favoured due to secondary orbital overlap. The exo product is thermodynamically favoured as it has less steric hindrance. These factors were previously discussed in Exercise 2 under section 3.4. Considering the cheletropic reaction and both Diels Alder reactions, the cheletropic TS has a higher energy than the Diels Alder TS, as it is more planar and there are strong eclipsing interactions developing between the aromatic 6-membered ring and the sulphone oxygen atoms which destabilises the TS.[11] However, the cheletropic product is more stable than both Diels-Alder products. Although a 5-membered ring typically suffers from more torsional strain than 6-membered rings[12], in this case the presence of a large sulfur atom would cause more distortions in the 6-membered ring. Thus, it can be concluded that the cheletropic pathway is under thermodynamic control while the Diels-Alder pathway is under kinetic control. The cheletropic product will be formed in equillibrating conditions.
IRC Analysis
2) Visualise the reaction coordinate with an IRC calculation for each path. Include a .gif file in the wiki of these IRCs.
The following figures illustrate the approach trajectory between o-xylylene and SO2 for the endo, exo and cheletropic reactions:

Xylylene is highly unstable. Look at the IRCs for the reactions - what happens to the bonding of the 6-membered ring during the course of the reaction?
o-xylylene is unstable due to the presence of 2 dienes locked in an s-cis conformation, which can act as good dienes for Diels-Alder reactions. All the carbons are sp2 hybridised, thus the molecule is planar. As SO2 approaches o-xylylene from either the top or bottom face, the 6-membered ring becomes aromatic due to it having 6 electrons. It is further evidenced by measuring the C-C bond lengths, which lie between an sp2C-sp2C and sp3C-sp3C bond at 1.40 Angstroms. This has a stabilising effect.
Extension
There is a second cis-butadiene fragment in o-xylylene that can undergo a Diels-Alder reaction. If you have time, prove that the endo and exo Diels-Alder reactions are very thermodynamically and kinetically unfavourable at this site.
The reaction between SO2 and the second cis-butadiene fragment is described in the scheme below. Similarly, endo and exo products are possible.
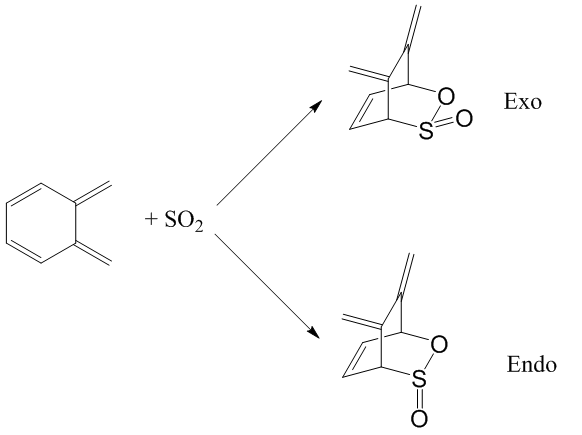
In a similar way as Exercise 3, the reactants, TS and products are optimised with Gaussian at PM6 energy level and the table below summarises their energies.
| Xylylene | SO2 | Endo TS | |||
|---|---|---|---|---|---|
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.178044 | 467.454558 | -0.118614 | -311.4210807 | 0.102070 | 267.98480541 |
| Endo Product | Exo TS | Exo Product | |||
| Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) | Energy (Hartrees) | Energy (kJ mol-1) |
| 0.065609 | 172.25644262 | 0.105054 | 275.81929801 | 0.067306 | 176.711916 |
Accordingly, the reaction barriers and reaction energies were calculated as follows:
| Reaction | Formation of Endo Product | Formation of Exo Product |
|---|---|---|
| Reaction barrier/ kJ mol-1 | +107.9 | +115.7 |
| Reaction energy/ kJ mol-1 | +12.2 | +16.6 |
Both Diels-Alder pathways on this cis-butadiene fragment proceed with an activation energy barrier that is much larger than if it proceeded with the former cis-butadiene fragment in Exercise 3. Moreover, is positive. This suggests that both reaction pathways at this cis-butadiene fragment are not feasible.
(Good extension Tam10 (talk) 13:39, 10 November 2017 (UTC))
Conclusion
The reaction dynamics of several Diels-Alder and a cheletropic reaction were analysed. From an optimisation of the atom coordinates in the reactants, transition state and products using Gaussian, various physical parameters (C-C bond lengths, free energies, MO energies) were extracted. In addition, an internal reaction coordinate (IRC) calculation was also run to visualise the trajectory of the reactants towards each other and how these physical parameters change along the reaction coordinate. The results obtained agreed with theory, in particular the Frontier Molecular Orbital theory, concerted mechanism of Diels-Alder reactions and the Endo Rule arising from secondary orbital interactions. The results also demonstrated that when SO2 is used as a dienophile, the Diels-Alder pathway is kinetically controlled while the cheletropic pathway is thermodynamically controlled.
Log Files
Exercise 1
| Species or IRC | Basis Set |
|---|---|
| PM6 | |
| Butadiene | File:BUTADIENEWITHMO CYY113 2.LOG |
| Ethene | File:ETHENE WITHMO CYY113.LOG |
| Butadiene/Ethene TS | File:TS WITHMO CYY113.LOG |
| Cyclohexene | File:Cyclohexeneproduct cyy113.log |
| IRC | File:CYCLOHEXENE TS IRC3 CYY113.LOG |
Exercise 2
| Species or IRC | Basis Set | |
|---|---|---|
| PM6 | BY3LYP/6-31(d) | |
| Cyclohexadiene | File:CYCLOHEXADIENE MIN cyy113.LOG | File:CYCLOHEXADIENE MIN 631 CYY113.LOG |
| 1,3-dioxole | File:DIOXOLE MIN cyy113.LOG | File:DIOXOLE631 2 cyy113.LOG |
| Endo TS | File:TS ex2 endo cyy113.LOG | File:Endots631mo cyy113.log |
| Exo TS | File:TS ex2 exo cyy113.LOG | File:Exots631mo2 cyy113.log |
| Endo Product | File:Endo PRODUCT MIN cyy113.LOG | File:ENDOPRODUCT 631 CYY113.LOG |
| Exo Product | File:ExoPRODUCT MIN cyy113.LOG | File:EXOPRODUCT631 CYY113.LOG |
Exercise 3
| Species or IRC | Basis Set |
|---|---|
| PM6 | |
| o-xylylene | File:XYLENE3 cyy113.LOG |
| SO2 | File:SO2 CYY113.LOG |
| Endo TS | File:EX3ENDOTS CYY113.LOG |
| Exo TS | File:XYLYLENE exoex3 TS cyy113.LOG |
| Cheletropic TS | File:INDENE TS cyy113.LOG |
| Endo Product | File:ENDOPRODUCT cyy113ex3.LOG |
| Exo Product | File:EXOPRODUCT OPT CYY113.LOG |
| Cheletropic Product | File:INDENEPRODUCT CYY113.LOG |
| Endo IRC | File:EX3ENDOTSIRC cyy113.LOG |
| Exo IRC | File:Xylene exo ts irccyy113.log |
| Cheletropic IRC | File:INDENEIRC CYY113.LOG |
Extension
| Species or IRC | Basis Set |
|---|---|
| PM6 | |
| o-xylylene | File:Xylene cyy113.log |
| SO2 | File:SO2 CYY113.LOG |
| Endo TS | File:Endotsextracyy113.log |
| Exo TS | File:Exotsextra cyy113.log |
| Endo Product | File:Endoproductmin4cyy113.log |
| Exo Product | File:EXOPRODUCTMIN4cyy113.LOG |
References
- ↑ 1.0 1.1 1.2 P. Atkins and J. De Paula, Atkins' Physical Chemistry, University Press, Oxford, 10th edn., 2014.,pp 470, 908-909
- ↑ 2.0 2.1 E. Anslyn and D. Dougherty, Modern physical chemistry, University Science, Sausalito, Calif., 2004.,pp. 896
- ↑ 3.0 3.1 R. Pearson, Accounts of Chemical Research, 1971, 4, 152-160.
- ↑ J. F. Chiang and S. H. Bauer, Journal of the American Chemical Society, 1969, 91, 1898–1901
- ↑ E. V. Anslyn and D. A. Dougherty, Modern physical organic chemistry, Univ. Science Books, Sausalito, CA, 2008, pp.22
- ↑ A. Bondi, The Journal of Physical Chemistry, 1964, 68, 441-451.
- ↑ 7.0 7.1 7.2 B. Oliveira, Z. Guo and G. Bernardes, Chem. Soc. Rev., 2017, 46, 4895-4950.
- ↑ 8.0 8.1 J. García, J. Mayoral and L. Salvatella, European Journal of Organic Chemistry, 2004, 2005, 85-90.
- ↑ B. Levandowski and K. Houk, The Journal of Organic Chemistry, 2015, 80, 3530-3537.
- ↑ R. Macomber, Organic chemistry, University Science Books, Sausalito, 1st edn., 1996.,pp. 248
- ↑ N. Isaacs and A. Laila, Tetrahedron Letters, 1976, 17, 715-716.
- ↑ G. Odian, Principles of polymerization, Wiley-Interscience, Hoboken, NJ, 2004.