Rep:Mod:ts Zh3615
This page was created by Zehua Hu, for third year computational laboratory Transition States and Reactivity.
Introduction
Computational Chemistry is a commonly adopted method to optimize and calculate molecular structures and variations of their properties during a reaction because of its theoretical accuracy and cost-efficiency. Commonly the optimization are achieved by calculating the first and second derivative of the potential energy surface (PES). The PES is obtained by plotting the potential energy V(q1, q2, ..., qn) against order parameters q1, q2, ..., qn (where n=3N-6, N=number of atoms) for each reaction. The reactant(s) and product(s) of each reaction are the minimums of the PES respectively and the transition state(s) is the saddle point(s) of the PES between the reactant(s) and product(s). Knowing the whole PES is concave, the reactant(s) and product(s) hence have zero first derivative dV/dqi and positive second derivative dV2/dqi2 while the transition state(s) has zero first derivative dV/dqi and negative derivative dV2/dqi2.
Nf710 (talk) 22:05, 29 November 2017 (UTC) The TS only has this in 1 demensions there rest have positive curvature
In 1-dimension, with approximating the chemical bonds between atoms to harmonic oscillators, the vibration modes of chemical bonds then obey the Hooke's Law (eq.1), and the vibrational frequencies (ω) of harmonic oscillators obey the (eq.2):
Equation 1:
Equation 2:
Where V is the elastic potential energy, k is the characteristic constant, x is the relative displacement of one atom to its connected atoms, m is the mass(for spring-mass model) or reduced mass (for atom-atom model). d2V/dx2=k can be obtained by differentiating the equation 1 twice, since the dV2/dqi2 is positive for reactant(s) and product(s) and negative for transition state(s) as suggested above, all vibrational frequencies are positive for reactant(s) and product(s) while a order parameter q that passes through the transition state produces one imaginary vibrational frequency. The only one imaginary vibrational frequency hence can be considered as the criteria for a successfully optimized transition state for multiple-transition-state reactions (e.g. exo- and endo-transition states).
In this computational laboratory, Gaussian worked similarly but in multiple dimensions to optimize and calculate reactant/product structures (min) and transition state structures (saddle point), relevant data was also obtained from the results of calculation and optimization e.g. bond lengths, MOs and energies. The less accurate semi-empirical method PM6 was applied for quick calculation and the DFT method BY3LYP/6-31(d) was used to obtain refined data e.g. for energy calculations.
Nf710 (talk) 22:11, 29 November 2017 (UTC) Good understanding of the vibrations
Butadiene and Ethylene
Procedure: The structures of butadiene and ethylene were drawn in Gaussview. Optimized structures, MOs and corresponding bond lengths of reactants were obtained from optimizing to PM6 level. The optimized structure of transition state was obtained by drawing guessed structures, optimizing to minimum (PM6), then optimizing to transition state (PM6, Berny, calculating force constancts for once) allowing Opt=NoEigen after freezing the bond lengths between two reaction sites of two alkenes to lengths of corresponding literature range, followed by IRC analysis (PM6). IRC analyzed structure i.e. the product cyclohexene was then optimized (PM6) to minimum to obtained optimized reaction product structure. Transition state was confirmed with frequency calculation (only 1 imaginary frequency) and IRC(correct shape).


| Butadiene (minimum) |
Ethylene (minimum) |
Transition state (T.S. Berny) |
IRC analysis |
Cyclohexene (minimum) |
|---|---|---|---|---|
| File:Butadiene zh3615.LOG | File:Ethylene zh3615.LOG | File:1tstots zh3615.LOG | File:1tstoirc zh3615.LOG | File:1ptomin zh3615.LOG |
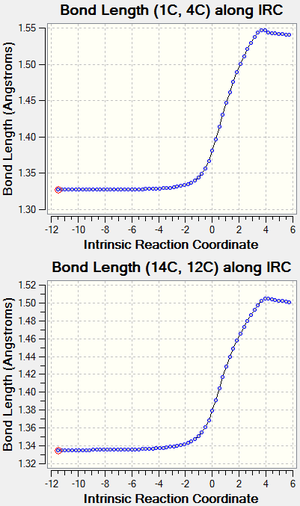
The carbon-carbon bonds lengths and their variation during the reaction are shown in the figure 1.1 and the literature bond lengths are shown in the figure 1.2, all obtained values are in literature range. The Van Der Waals radius of carbon atoms is 1.7 Å. [2] Detailed variation of bond lengths with time is shown in the table 1.1. As the reaction proceeds,the bond lengths of double bonds in both alkenes increases as they are gradually transformed to single carbon-carbon bonds, the bond length of the only single bond in the butadiene decreases as it was transformed to a double carbon-carbon bond gradually. The forming single carbon-carbon bonds of transition state between two alkenes have a bond length of 2.115 Å, which are shorter than two times of Van der Waals radius of carbon (3.4 Å).
(Fv611 (talk) You are not comparing the value of the bonds being formed at the TS with a typical sp3-sp3 bond length.)
| |
 |

|
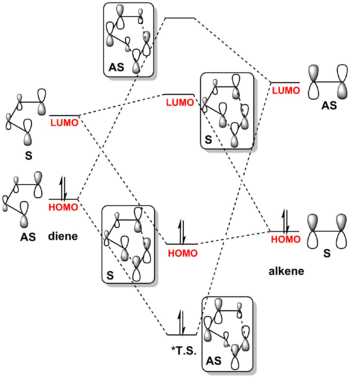
MO diagram (fig 1.3) was constructed with symmetry labels in normal demand DA according to the relatively heights of MOs calculated. HOMOs-LUMO region orbitals were visualized in table 1.2 and 1.3. *NB height of each T.S. MO is the height of corresponding product. (Fv611 (talk) It is unclear what you mean by height, or corresponding product.)
(Fv611 (talk) Your MO combinations are correct, but your energy levels are wrong. As the JMols are not included, as as you have not specified why they are not included, I cannot tell whether you have calculated the MOs correctly. In this reaction, both HOMOs of the reactants are lower in energy than the TS HOMO-1, and the ethene LUMO is higher in energy than the TS LUMO+1. Additionally, you have not labelled your MOs consistently between your table with the pictures and the MO diagram.)
The energy gap between HOMO and LUMO is smaller for butadiene due to its stronger conjugation system. The HOMO and LUMO of the transition state is formed by the interaction between the HOMO of ethylene and LUMO of butadiene. Both the reacting orbitals and formed orbitals are symmetric, which indicates that the reacting orbitals in the allowed Diels-Alder reaction for 6-pi-electron system should have the same symmetry. It can be mathematically demonstrated by observing the equation of wavefunction overlap integral (S) (eq. 4) below:
Equation 4:
Where ψa and ψb are the wavefunctions of reactant MOs, for these MOs to react, they must have non-zero wavefunction overlaps. S is non-zero for S-S, AS-AS interactions and is zero for S-AS and AS-S interactions. Hence Diels-Alder reaction is allowed for MOs of the same symmetry and forbidden for the MOs of different symmetries.
| LUMO | HOMO | |
|---|---|---|
| Butadiene MO |  |
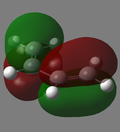
|
| Butadiene MO Visualization |
 |
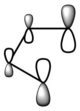
|
| Ethylene MO | 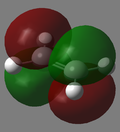 |

|
| Ethylene MO Visualization |
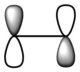 |

|
| LUMO+1 | LUMO | HOMO | HOMO-1 | |
|---|---|---|---|---|
| T.S. MO |  |
 |
 |
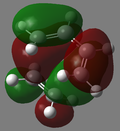
|
| T.S. MO Visualization |  |
 |
 |

|
As shown in the IRC analysis in table 1.4, the vibration that passes through the transition state is -949 cm-1, the variation of the bond lengths of forming bond is identical in shape and value, which indicates that the new c-c single bonds are made simultaneously during the approaching of alkenes. In contrast, asynchronous formation of new bonds will produce different bond length curves between two forming bonds.
| Transition state vibration | Transition state animation |
|---|---|
 |
|

| |

| |
Cyclohexadiene and 1,3-Dioxole
Procedure:The structures of cyclohexa-1,3-diene, 1,3-dioxole and their corresponding Diels-Alder products via exo- and endo- transition states were drawn in Gaussview. Optimized structure were obtained by optimizing to PM6 level. The optimized transition states were obtained by breaking the formed bond in the D-A reaction, then optimizing to transition state (PM6, Berny, calculating force constants for once) allowing Opt=NoEigen after freezing the bond lengths between two reaction sites of two alkenes to lengths of corresponding literature range, followed by IRC analysis (PM6), where MOs of transition states were also obtained. Orders of MOs via each reaction pathway were generated from the single point energy calculation. Each calculation was then further optimized to B3LYP/6-31G(d) level for the calculation of the reaction barriers and reaction energies at room temperature. Obtained transition states were confirmed with frequency calculation (only 1 imaginary frequency).
| Cyclohexadiene (minimum,PM6) |
Cyclohexadiene (minimum,B3LYP) |
1,3-dioxole (minimum,PM6) |
1,3-dioxole (minimum,B3LYP) |
|---|---|---|---|
| File:Hextominpm6 zh3615.LOG | File:Hextomin631 zh3615.LOG | File:Diotominpm6 zh3615.LOG | File:Diotomin631 zh3615.LOG |
| Exo-T.S. (T.S. Berny,PM6) |
Exo-T.S. (T.S. Berny,B3LYP) |
Exo-T.S. IRC | Exo-T.S. energy analysis |
| File:2EXOtoTSpm6 zh3615.LOG | File:2EXOtoTS631 zh3615.LOG | File:2EXOtoIRC zh3615.LOG | File:2EXOtoENERGY zh3615.LOG |
| Endo-T.S. (T.S. Berny,PM6) |
Endo-T.S. (T.S. Berny,B3LYP) |
Endo-T.S. IRC | Endo-T.S. energy analysis |
| File:2ENDOtoTSpm6 zh3615.LOG | File:2ENDOtoTS631 zh3615.LOG | File:2ENDOtoIRC zh3615.LOG | File:2ENDOtoENERGY zh3615.LOG |
| Exo-reaction product (minimum,PM6) |
Exo-reaction product (minimum,B3LYP) |
Endo-reaction product (minimum,PM6) |
Endo-reaction product (minimum,B3LYP) |
| File:Exoproductpm6 zh3615.LOG | File:EXOproduct631 zh3615.log | File:Endoproductpm6 zh3615.LOG | File:ENDOproduct631 zh3615.log |
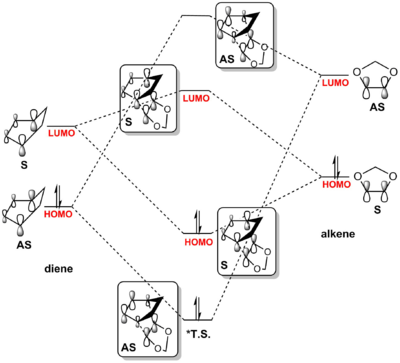
The MO diagram of this reaction was drawn according to the MO order calculated from energy analysis in the Gaussview as shown in table 2.1 following the rule in the figure 1.3. The energy gap between the LUMO of diene and the HOMO of alkene was calculated to be smaller than the energy gap between the HOMO of diene and LUMO of alkene. It suggested that both reactions are reverse demand Diels-Alder reactions. In a normal demand D-A reaction the HOMO of diene reacts with the LUMO of the alkene while the HOMO of the alkene reacts with the LUMO of diene in an inverse demand D-A reaction. In both reactions, the HOMO of alkene react with the LUMO of diene,
(Fv611 (talk) The HOMO of the diene reacting with the LUMO of the dienophile does not prevent the LUMO of the diene from reacting with the HOMO of the dienophile. In fact, in both this exercise's and the previous exercise's reactions both of this pairing. However this reaction is inverse electron demand whereas the reaction between butadiene and ethene is normal demand. The difference is given by whether the diene's or the dienophile's HOMO has the lowest energy, and on which LUMO has the highest energy. Your reactant's FMO's have the correct energies, but your TS MO's relative energies are not correct. Additionally, you correctly say that the symmetry of the TS MOs is A-S-S-A (for HOMO-1, HOMO, LUMO and LUMO+1 respectively) but you show in table 2.3 a symmetric LUMO+1 and an antisymmetric LUMO for the exo case. It would also have been nice to include a comment on the difference between the exo and endo case.)
The HOMO-LUMO region orbitals of the endo- and exo- transition states were visualized and shown in two different angle (for view of overlap and symmetry respectively) in the table 2.2. The activation energies, reaction energies and other related data were calculated and tabulated in table 2.3.
| MO diagram via exo-T.S. | MO diagram via endo-T.S. |
|---|---|
 |
 |
| Exo- transition state (side view) |
Exo- transition state (top view) |
Endo- transition state (side view) |
Endo- transition state (top view) | |
|---|---|---|---|---|
| LUMO+1 | 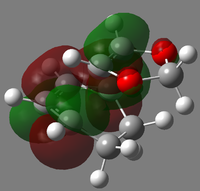 |
 |
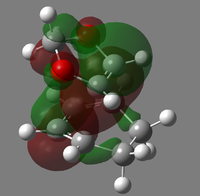
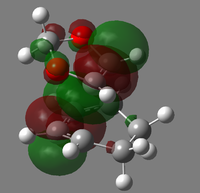 |

|
| LUMO | 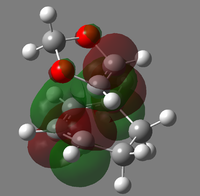 |
 |
 |

|
| HOMO |  |
 |
 |

|
| HOMO-1 | 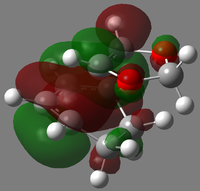 |
 |
 |

|
| via endo-Diels-Alder T.S. | via exo-Diels-Alder T.S. | |
|---|---|---|
| Cyclohexadiene | 306.9 (-612593) | |
| 1,3-dioxole | -137.3 (-701187) | |
| Reactant sum | 169.6 (-1313781) | |
| Transition state | 362.2 (-1313622) | 364.7 (-1313614) |
| Reaction product | 99.2 (-1313849) | 99.7 (-1313846) |
| Activation energy | 192.6 (159.0) | 195.1 (167.0) |
| Reaction energy | -70.3 (-68.0) | -69.9 (-64.0) |
As shown in the table 2.3, both the activation energy and reaction energy via endo-transition state is less compared to the Diels-Alder reaction via exo-transition state, which indicates the endo-reaction product is both the kinetic and thermodynamic product. It is because of the favourable secondary orbital interactions during the formation of endo-product (lower the activation energy) and steric clashes in the exo-product (make the exo-product less thermodynamically stable), both effects are observable by comparing the HOMOs of transition states.
Nf710 (talk) 22:15, 29 November 2017 (UTC) This is a nice and consise section. However I think you could have gone into a bit more detail. Also it would have been nice if you had shown some more understanding of the quantum chemical methods going on. Also you energies seem to be slightly out.
O-Xylene-So2 Cycloaddition
Procedure:The structure of 5,6-dimethylenecyclohexa-1,3-diene, sulphur dioxide and their corresponding products via exo-Diels-Alder, endo-Diels-Alder and cheletropic transition states were drawn in Gaussview. The optimized structures were obtained by optimizing to PM6. The optimized transition states were obtained by breaking the formed bond in each reaction, then optimized to transition state (PM6, Berny, calculating force constants for once), allowing Opt=NoEigen after freezing the bond lengths to lengths of corresponding literature range between two reaction sites of two alkenes followed by IRC analysis (PM6).

| O-Xylene(minimum,PM6) | SO2(minimum,PM6) | |
|---|---|---|
| File:Oxypm6zh3615.LOG | File:So2pm6zh3615.LOG | |
| Exo-D-A-T.S.(T.S. Berny,PM6) | Exo-D-A-T.S.(IRC analysis) | Exo-D-A-product(minimum,PM6) |
| File:Daexototstspm6.log | File:Daexoirc zh3615.log | File:Daexoproductminpm6.LOG |
| Endo-D-A-T.S.(T.S. Berny,PM6) | Endo-D-A-T.S.(IRC analysis) | Endo-D-A-product(minimum,PM6) |
| File:Daendototstspm6.log | File:Daendoirc zh3615.log | File:Daendoproductminpm6.log |
| Cheletropic-T.S.(T.S. Berny,PM6) | Cheletropic-T.S.(IRC analysis) | Cheletropic-product(minimum,PM6) |
| File:Chetotstspm6.log | File:CHE IRC zh3615.log | File:Cheproductpm6 zh3615.LOG |
Each reaction was visualized by carrying out IRC analysis at the PM6 level as shown in the table 1.6. Activation energies and reaction energies were calculated as shown in the table 1.7, and energy profiles are shown in figure 3.2.
| Endo-Diels-Alder T.S. | Exo-Diels-Alder T.S. | Cheletropic T.S. | |
|---|---|---|---|
| Animation |  |
 |
|
| Animation | 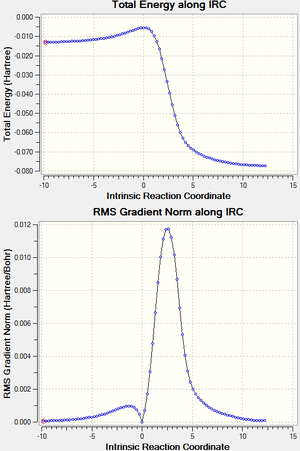 |
 |
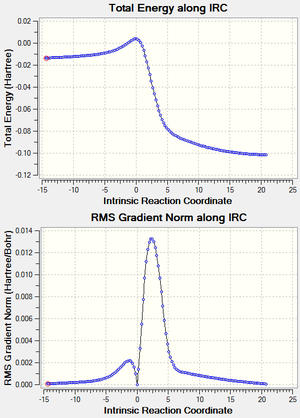
|
All carbons in o-xylylene is sp2 hybridised, which makes o-xylylene a planar molecule that can be attacked at the cis diene easily from both sides. During the approaching of SO2 molecule, the 6-membered ring quickly becomes 6 pi aromatic system by resonance with the two double bonds adjacent to the 6-membered ring. The structure of o-xylylene is changed due to the stabilization from aromatic system.
| via endo-Diels-Alder T.S. | via exo-Diels-Alder T.S. | via cheletropic T.S. | |
|---|---|---|---|
| O-xylylene | 467.2 | ||
| Sulphur dioxide | -311.4 | ||
| Reactant sum | 155.8 | ||
| Transition state | 237.8 | 241.8 | 260.1 |
| Reaction product | 57.0 | 72.1 | -0.00525 |
| Activation energy | 82.0 | 86.0 | 104.3 |
| Reaction energy | -98.8 | -83.7 | -155.8 |
Among three reaction pathway, the most kinetically stable product is endo-Diels-Alder product (due to secondary orbital interactions) and the most thermodynamically stable product is cheletropic product (less steric hindrance between the 6-membered ring and sulphone oxygen compared to both Diels-Alder products), the ring strain is also not significant due to the size of sulphur atom.

(Just for the sake of communication, it's useful to have the energy differences on reaction profile diagrams Tam10 (talk) 11:32, 24 November 2017 (UTC))
Extension
The Diels-Alder reaction between o-xylylene and SO2 is possible but unfavourable in theory. The protocol in the section 3 was followed to calculate the reaction barriers and reaction energies of the below reaction as shown in the table 4.1.

| via endo-Diels-Alder T.S. | via exo-Diels-Alder T.S. | |
|---|---|---|
| O-xylylene | 467.2 | |
| Sulphur dioxide | -311.4 | |
| Reactant sum | 155.8 | |
| Transition state | 268.0 | 275.8 |
| Reaction product | 172.3 | 176.7 |
| Activation energy | 95.7 | 99.1 |
| Reaction energy | 16.5 | 20.9 |
The reaction barrier and reaction energy is both positive and relatively large compared to the reaction at the other diene fragment in section 4, which indicates that this reaction are not spontaneous and are not likely to occur under normal conditions.
| Exo-D-A-T.S.(T.S. Berny,PM6) | Exo-D-A-product(minimum,PM6) |
|---|---|
| File:EXOTSTOTS444zh3615.LOG | File:EXOPRODUCTMIN444zh3615.LOG |
| Endo-D-A-T.S.(T.S. Berny,PM6) | Endo-D-A-product(minimum,PM6) |
| File:ENDOTSTOTS444ZH3615.LOG | File:ENDOPRODUCTMIN444zh3615.LOG |
Conclusion
The structures of reactants, product and transition states of three Diels-Alder reactions were determined using GaussView 5.0.9. Semi-empirical method PM6, density functional theory method (DFT) B3LYP, IRC analysis and energy analysis were adopted in the optimization, visualisation and calculation. Theoretical values of bond lengths, MO order, sum of electronic and thermal free energies etc. were obtained and the competition between Dies-Alder reaction and Cheletropic reactions was investigated. All obtained data agreed with theory or was in literature range.
Reference
[1] E. V. Anslyn and D. A. Dougherty, Modern physical organic chemistry, Univ. Science Books, Sausalito, CA, 2008, pp.20-22
[2] A. Bondi, The Journal of Physical Chemistry, 1964, 68, 441-451.
