Rep:Mod:ts VHO
Introduction
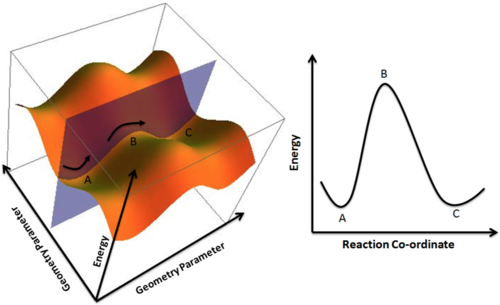
The potential energy surface shows all possible reaction pathways to get from reactant A to product C. The transition state is more likely to take the path involving the lowest energy and normal to the reaction path. The highest energy state of the path taken is the transition state. The curvature of the transition state is a local maximum, the result is a negative frequency due to imaginary number whereby i is equal to square root of -1. All minima and transition states have zero gradient with respect to the potential energy surface. Global minimum is a minimum on potential energy surface, this point is where the energy is lowest for all possible reaction pathways. At the global minimum, the structure is most thermodynamically stable and thus the most favorable structure. Local minimum is also minimum on a potential energy surface, this minimum is usually for a range of coordinates (Reactant A or Product C).
Nf710 (talk) 14:00, 16 April 2017 (BST) You should have explained with second derivatives here. The reaction coordinate is the coordinate of the TS which has negative curvature. All all dimensions of the PES at the TS have positive (2nd derivative) curvature.
Exercise 1: Reaction of Butadiene with Ethene
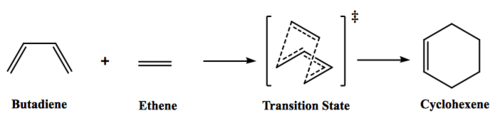
Reaction of butadiene and ethene will be computationally modelled in this exercise. This is a [4+2]-cycloaddition, involving breaking of three π bonds and forming two new σ bonds with one new π bond.
- MO Diagram for Formation of Butadiene/Ethene Transition State
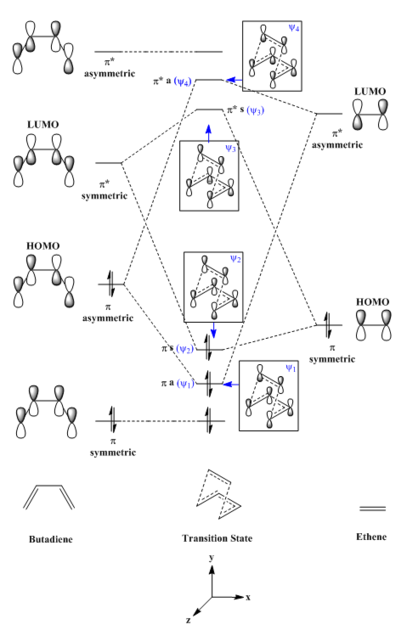
(Fv611 (talk) 16:10, 5 April 2017 (BST) Good MO diagram, but you switched around the schematic MOs for HOMO and LUMO+1.)
- LUMO and HOMO for butadiene and ethene
| Butadiene | Ethene | ||||||
|---|---|---|---|---|---|---|---|
|
| ||||||
|
|
- MOs for Tranisition State (ψ1, ψ2, ψ3 and ψ4)
| Transition state |
|
|
|
|
|---|
The MO diagram shows four molecular orbitals in transition state are formed from butadiene and ethene frontier orbitals of the same symmetry and close in energy. Asymmetric (π butadiene HOMO (MO 11) combines with asymmetric (π* ethene LUMO (MO 7) forming a pair of asymmetric molecular orbitals, ψ1 (MO 16) and ψ4 (MO 19) in the transition state. Conversely, symmetric (π* butadiene LUMO (MO 12) combines with symmetric (π ethene HOMO (MO 6) forming a pair of symmetric molecular orbitals, ψ2 (MO 17) and ψ3 (MO 18) in the transition state. However, this is not expected for a normal demand of Diels-Alder reaction. The energy gap between the two symmetric (π) frontier orbitals (LUMO of butadiene and HOMO of ethene) is very large compared to the energy gap between the two asymmetric (π*) frontier orbitals (HOMO of butadiene and LUMO of ethene). Thus, the interaction is dominated by combination of HOMO of butadiene and LUMO of ethene frontier orbitals.
The orbital symmetry requirement of same symmetry can be explained from quantum mechanics, whereby the overlap integral, SAB, is the product of the wavefunction and its complex conjugate, which quantifies the orbital interaction.
where A and B are arbitrary atoms
The product of two terms with same symmetry (symmetric-symmetric or asymmetric-asymmetric) results in symmetric, leading to orbital interaction as the integral is non-zero. The integral will equal zero when the product of two terms do not have same symmetry (asymmetric-symmetric), hence no orbital interaction between the orbitals.
As mentioned previously, the four MOs (ψ1, ψ2, ψ3 and ψ4) formed in transition state are derived from two frontier orbitals of same symmetry. From the transition state MOs, it can be seen that ψ1 and ψ2 are formed from frontier orbitals of the same phase, resulting in greater bonding interaction and lower in energy which can be seen in the MO diagram. By observation of the transition MOs for ψ3 and ψ4, it can be seen there are nodes due to change of phase as it is an antibonding interaction, hence higher in energy which can also be observed from MO diagram.
ψ1 HOMO -1 (MO 16) = HOMO (MO 11) + LUMO (MO 7)
ψ2 HOMO -1 (MO 17) = LUMO (MO 12) + HOMO (MO 6)
ψ3 HOMO -1 (MO 18) = LUMO (MO 12) + HOMO (MO 6)
ψ4 HOMO -1 (MO 19) = HOMO (MO 11) + LUMO (MO 7)
Bond Length Analysis
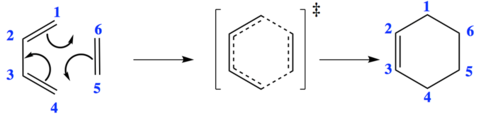
- Mechanism of Diels-Alder reaction of butadiene and ethene
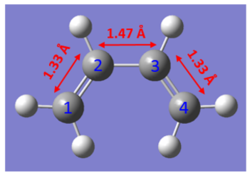
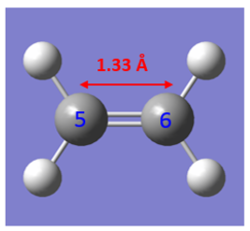
- Table of Experimental C-C Bond Lengths
| Butadiene | Ethene | Cyclohexene |
|---|---|---|
 |
 |
 |
The C-C bond lengths of the reactants and product are obtained from computational calculations. As expected from the mechanism shown in Figure 4, three double bonds (C1-C2, C3-C4 and C5-C6) are broken, and forming one double bond (C2-C3) and two single bonds (C1-C5 and C6-C4). For butadiene molecule, a single C-C bond length is 1.33 Å and double C-C bond length is 1.47 Å. For ethene molecule, bond length of double bond I s 1.33 Å.
- Table of Typical C-C Bond Lengths
| Type of C-C Bond | Typical Length (Å) |
|---|---|
- Transition State C-C Bond Lengths
| Transition State |
|---|
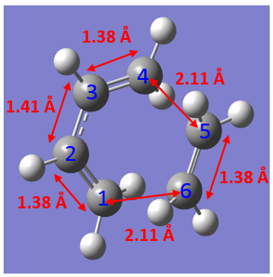 |
All the C-C bonds of the reactants becomes the intermediate, initially the double bond of ethene shortens and terminal bonds lengths whilst the central bond shortens of butadiene forming the transition state. Bond lengths for C4-C5 and C6-C1 are calculated to be 1.54 Å, this is less than twice of the Van der Waals radius. This suggests that there is bonding interaction between butadiene and ethene.
(Fv611 (talk) 16:10, 5 April 2017 (BST) Unfortunately, the comparison to make was between the Van der Waals radius of carbon and the bond length at the transition state, not that of the product (which logically will match the typical sp3-sp3 bond length.)
Vibration of Transition State
| Transition State Vibration | ||
|---|---|---|
The animation of the reaction path of transition state shows the formation of two single C-C bonds forming in a synchronous process. The frequency of the vibration is -949.81 cm-1, this is negative since the vibration takes place at a maxima on the potential energy surface.
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
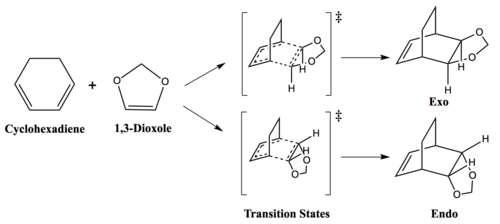
Diels-Alder reaction of cyclohexadiene and 1,3-dioxole forms two products, exo and endo, depending on the orientation of the interaction in the transition state. If cyclohexadiene overlaps with 1,3-dioxole, this will result in exo-product with 1,3-dioxole pointing towards cyclohexadiene. Conversely, if 1,3-dioxole is pointing away from cyclohexadiene, this will result in endo-product. The MO diagram for the formation of cyclohexadiene/1,3-dioxole transition state will be the same for both exo and endo product.
Although the MO diagram of cyclohexadiene and 1,3-dioxole is similar to butadiene/ethene, this [4+2]-cycloaddition is an inverse electron demand Diels-Alder reaction as the dienophile contains electron donating groups (-OR). Since the HOMO and LUMO of dienophile energies increases, there is a strong dominating interaction between LUMO of diene and HOMO dienophile, resulting in the LUMO and HOMO of the transition state.
- MO Diagram for Formation of Cyclohexadiene/1,3-Dioxole Transition State
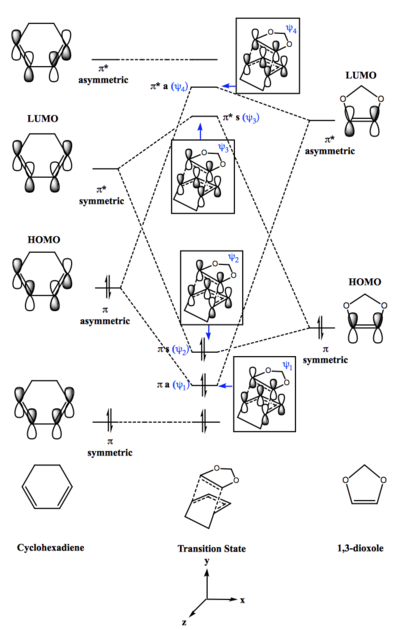
- LUMO and HOMO for Cyclohexadiene/1,3-Dioxole
| Endo |
|
|
|
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exo |
|
|
|
|
- Reaction Profile of Cyclohexadiene with 1,3-Dioxole
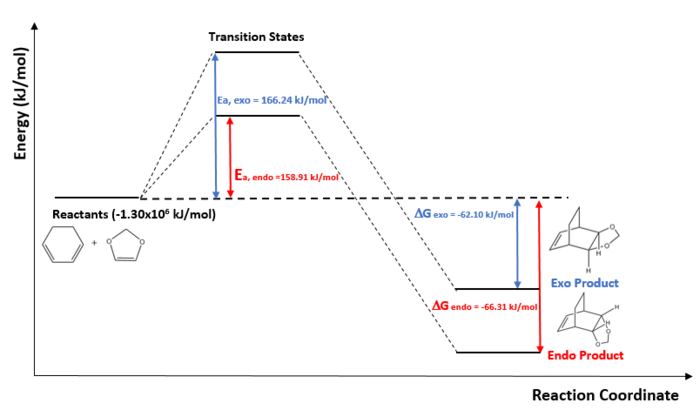
The endo-product is kinetically and thermodynamically favoured compared to exo-product since it has a lower activation energy and is a more stable product. The endo product is sterically less hindered which can be seen from the MOs. Secondary orbital interactions may also result to stabilisation of endo transitions state, thus the lower activation energy.
- Endo and Exo Product Transition State HOMO
| Endo TS HOMO | Exo TS HOMO | ||||
|---|---|---|---|---|---|
For the endo HOMO of the transition state, it can be observed that the oxygen atoms on dienophile are in-phase to the central C-C bond of diene whilst out of phase with the rest of the regions on the dienophile. This may be the dominating stabilisation effect on the transition state, thus the lower energy of 158.91 kJ/mol. For exo HOMO of the transition state, this is not observed so no interaction between the oxygen atoms and the central carbons.
Nf710 (talk) 14:08, 16 April 2017 (BST) Correct results, excellent diagram. Good explanation of the thermo and kenetic products.
Exercise 3: Diels-Alder vs Cheletropic
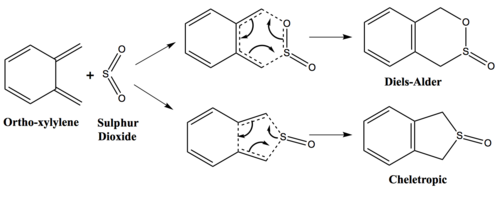
Pericyclic of ortho-xylylene with sulphur dioxide can proceed via two different reaction mechanism; Diels-Alder reaction and Cheletropic reaction. All three possible products; endo, exo and cheletropic, involves formation of two new bonds. As shown in the mechanism above, for Diels-Alder the two new bonds are C-O and C-S compared to Cheletropic with two new bonds formed with same sulphur atom.
- Animation of Reaction Coordinates
| Endo | Exo | Cheletropic |
|---|---|---|
  |
  |
 |
The bond formation for Diels-Alder involve two new bonds formed with two different heteroatoms (O and S) and for Cheletropic both bonds are formed with same S atom. Thus, Diels-Alder and Cheletropic are asynchronous and synchronous respectively.
- Reaction Profile of Ortho-xylylene with Sulphur Dioxide
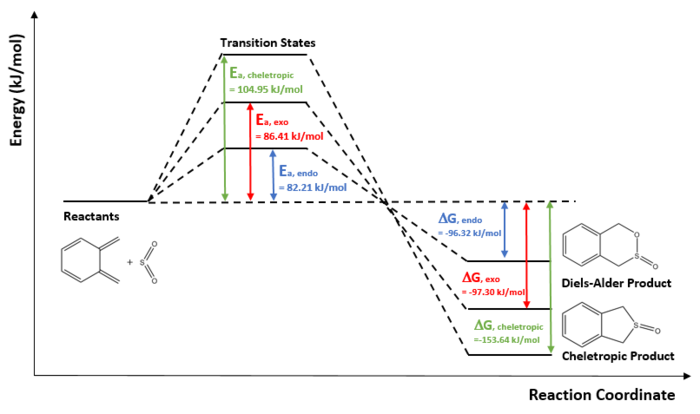
The reaction profile above shows the endo product is the kinetic product which is the same as previously for reaction of cyclohexadiene and 1,3-dioxole. This could be due to the secondary orbital interaction stabilisation in the transition state, between the oxygen from sulphur dioxide and the carbons from diene. However, the cheletropic product is the thermodynamically favoured.
(This section is a bit light on text. But the reaction profile is nice Tam10 (talk) 18:37, 3 April 2017 (BST))
Diels-Alder Reaction of Second Cis-Butadiene Fragment in Ortho-xylylene with Sulphur Dioxide
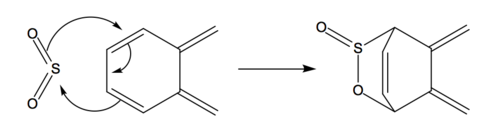
As shown in the mechanism above, Diels-Alder reaction can also take place at the second cis-butadiene fragment and resulting in endo and exo product.
- Reaction Profile
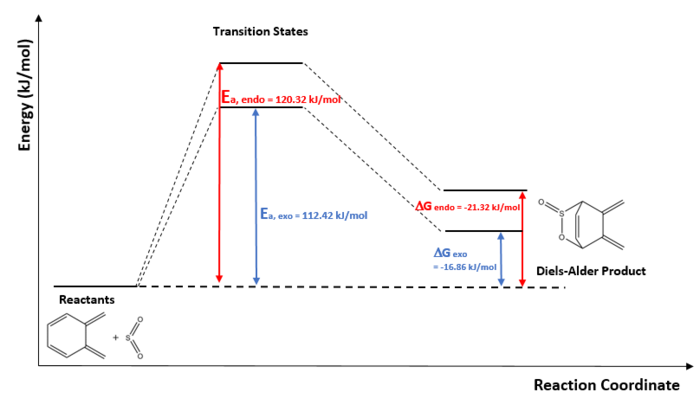
As shown in the reaction profile, the activation energies for endo and exo pathway is much greater compared to previous reaction profile activation energies. The reaction barrier is greater and thus kinetically unfavourable. This reaction is also thermodynamically unfavourable since the endo and exo products are both at a higher energy than the reactants, so the stability of formation is not increased. The reason for this could be the disruption of aromaticity during the reaction or the steric strain of the product.
Conclusion
Reaction pathways and transition states were studied for different Diels-Alder reaction via computational methods. Interpretation of the HOMO and LUMO of the reactants provides a better understanding of the transition states MOs electron density. By extrapolation and computational calculations we are able to produce reaction profile and conclude which product is kinetically or/and thermodynamically favourable.
