Rep:Mod:ts.scc215
Third Year Computational Lab: Transitional States and Reactivity
Introduction

A Potential Energy Surface (Fig. 1) is a landscape about that shows various reaction pathway of completing a reaction from reactants to materials. It always possesses dimension numbers of 3N-6 for N standing for the number of molecules involved.
Nf710 (talk) 10:23, 17 November 2017 (UTC) This is not correct N is the number of atoms in the molecule.
A minimum could be divided into two categories, local minima and global minimum under the context of PES. Multiple local minima could exist on the PES, and reactants are normally located on one of them, while global minimum on PES often refers to the location of the most thermodynamic stable product. For a local minimum, it satisfies the following prerequisites: and , which also defines minimum as one of the critical points of the function.
The transition state is one of the local maximum located on minimum energy pathway, and it is the position which most of the reaction pathways passes through. The derivative of the function at TS also showed since it is also one of the critical points of the function. . However, TS could be distinguished from local minimums since it has a negative second derivative and thus indicated a correctly optimized reactant and product should have all positive frequencies while a TS should show one imaginary frequency as the result.
Nf710 (talk) 10:28, 17 November 2017 (UTC) This is a nice intro but I am not sure if you fully understand. Do you know that the subscript i is in your equations? It is a normal mode so a minimum has positive second derivatives for all dimensions. A TS has positive second derivatives for all modes except the reaction coordinate.
Exercise 1: Reaction of Butadiene with Ethylene
In this reaction, a [4+2] cycloaddition was simulated on Gaussian and the interactions between frontier orbitals have been investigated to determine if the reaction is normal demand or inverse demand DA reaction.
MO diagram of frontier orbitals and qualitative energy levels of reaction

(Fv611 (talk) This MO diagram is incomplete, and partly wrong. An MO diagram should show which fragment orbitals are combined to form the TS MOs. If you had done that you would have picked up on the fact that you are combining the antisymmetric butadiene HOMO with the symmetric ethene HOMO to form a wrong TS HOMO, and the symmetric butadiene LUMO with the antisymmetric ethene LUMO to form a wrong TS LUMO+1.)
Using the energy levels listed in checkpoint files of molecules and transition states, a molecule diagram was produced. It has clearly shown that the reaction is normal demand since the gap between LUMO of butadiene and HOMO of ethylene is large such that the overlap between these two is poor. As a result, the reaction is completed through the domination of better-overlapped HOMO of butadiene and LUMO of ethylene. For such bond-forming reaction to proceed, the positive phase of molecular orbitals must overlap, i.e. only symmetric-symmetric reactions and asymmetric-asymmetric overlap are allowed and the rest are forbidden. This means the orbital overlap integrals of symmetric-symmetric and that of asymmetric-asymmetric interactions are non-zero while that of asymmetric-symmetric interactions are zero.
(Fv611 (talk) Actually, the symmetry requirements are given by the value of the orbital overlap, not the other way around.)
Variation of C-C bond length and their comparison to different types of C-C bond
| Bond Length (Å) | C1-C4 | C4-C5 | C5-C6 | C6-C11 | C11-C14 | C14-C1 |
|---|---|---|---|---|---|---|
| Butadiene | 1.335 | 1.468 | 1.335 | |||
| Ethylene | 1.331 | |||||
| Transition State | 1.379 | 1.411 | 1.379 | 2.115 | 1.381 | 2.115 |
| Product | 1.492 | 1.331 | 1.492 | 1.536 | 1.537 | 1.536 |
The two tables have summarized the change of bond length throughout the reaction. Three double bonds in original reactants (C1-C4, C5-C6, C11-C14) has increased the bond length to 1.492 Å and 1.537 Å respectively since their bond order has been reduced to 1 in the product. Bond C4-C5 has decreased in bond length since it has become a C-C double bond instead of a single bond. C6-C11 and C14-C1 are the two newly formed bonds in the product such that the distance between carbon atoms has decreased from 3.4 Å to 1.536 Å which is similar to the length of a sp3C-sp3 C bond.
(Fv611 (talk) You don't explicitly discuss the relationship between carbon VdW radii, the length of the bonds being formed at the transition state and the length of sp3-sp3 bonds.)
At TS, the originally existing bonds have their length located between 1.379 to 1.411 Å, which are between a pure sp3C-sp3C bond length and sp2C-sp2C bond length and also shorter than sp3C-sp2C bond.
| sp3C-sp3C Bond length (Å) | Error (Å) | sp2C-sp2C Bond length (Å) | Error (Å) |
|---|---|---|---|
| 1.526 (propane) or
1.525 (isobutane) |
0.002 | 1.338 | 0.002 |
| Carbon atom Van der Waals Radius: 1.700 Å | |||
| C-C bond | |||
|---|---|---|---|
| C1-C4 | 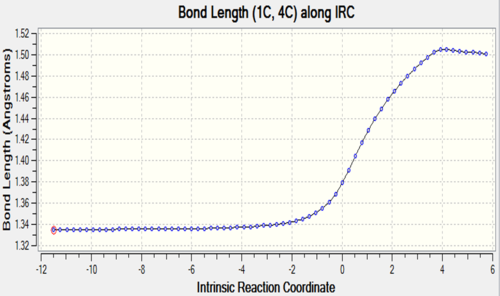
|
C6-C11 | 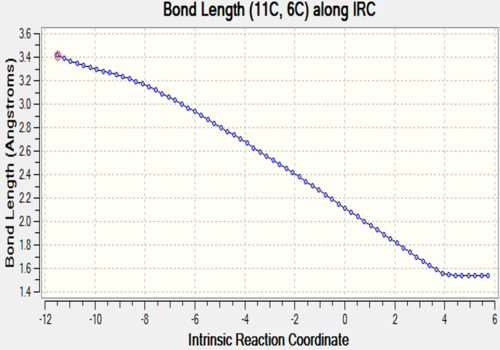
|
| C4-C5 | 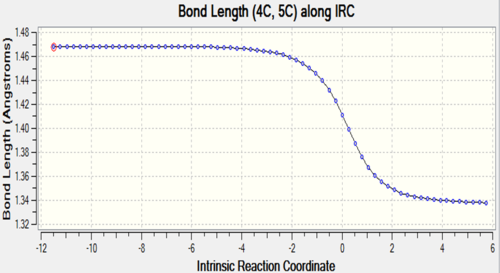
|
C11-C14 | 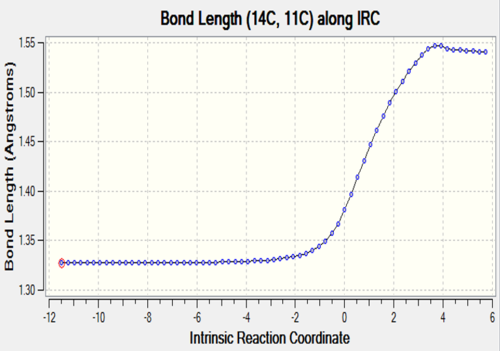
|
| C5-C6 | 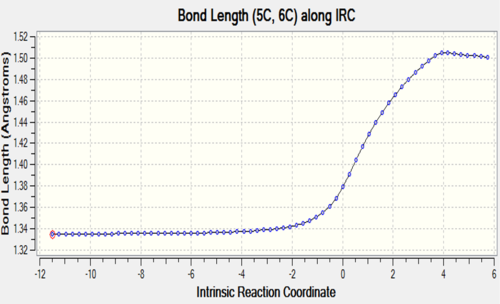
|
C14-C1 | 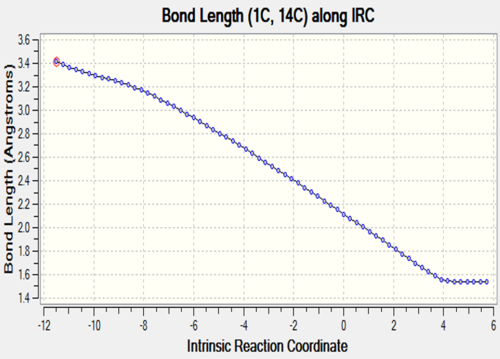
|
Vibration in Imaginary frequency of TS

From the processed simulation, only one single imaginary vibration frequency at 948.9i cm-1 was reported, which stands for the synchronous bond formation process during the Diels-Alder reaction.
(Fv611 (talk) There is no proof here that you have optimised the TS, as the LOG file you've attached ends abruptly.)
Calculation
File:Final irc scc215.logFile:FINISHED TS FOR IRC scc215.LOGFile:ETHENE scc215.LOGFile:BUTADIENE scc215.LOG
Exercise 2: Reaction between Cyclohexadiene and 1,3-dioxole



(You've made a few mistakes in the MO diagrams. You should link up the MOs to show how they are formed. Also the components that you use in the HOMO and LUMO+1 are the wrong symmetry and shouldn't interact Tam10 (talk) 16:24, 12 November 2017 (UTC))
MO diagram of Exo and Endo reaction pathways
Similar to all the other [4+2] cycloaddition, the reaction is controlled by the overlap of frontier orbitals of diene and dienophile molecule respectively. As discussed above, the overlap integral is non-zero (i.e. reaction allowed) only if the symmetrical element is identical. Hence, in this case, the overlap between LUMO of cyclohexadiene and HOMO of 1,3-dioxole dominates the reaction since the energy gap between is much smaller than the other energy gap between HOMO of cyclohexadiene and LUMO of 1,3-dioxole. Thus this reaction, regardless of endo or exo reaction, is an inverse electron demand DA reaction.
Which one is more favourable? Endo product vs Exo product
| Energies (Hatrees/molecule) | Cyclohexadiene | 1,3-dioxole | TS | Product | ||||
|---|---|---|---|---|---|---|---|---|
| Sum of electronic and thermal Free Energies of Endo reaction |
|
|
|
| ||||
| Sum of electronic and thermal Free Energies of Exo reaction |
|
|
|
|
Endo Reaction Energies
Activation Energy: [-500.395506-(-233.333434-267.068135)]*4.3597439422E-18/1000*6.02E-23 = 3.80459075 kJ/mol
Reaction Energy: [-500.436135-(--233.333434-267.068135)]*4.3597439422E-18/1000*6.02E-23 = -21.69049711 kJ/mol
Exo Reaction Energies
Activation Energy: [-500.348863-(-233.333434-267.068135)]*4.3597439422E-18/1000*6.02E-23 = 138.379614 kJ/mol
Reaction Energy: [-500.434789-(--233.333434-267.068135)]*4.3597439422E-18/1000*6.02E-23 = -87.2191166 kJ/mol
Nf710 (talk) 10:46, 17 November 2017 (UTC) I dont know what you have done here but your energies for both seem to be extremely out.
Explanation of the result
As shown in the calculated result, considering both thermodynamic and kinetic aspect, endo product formation is more favourable in this reaction than the exo form. This could be shown by the lower reaction barrier of endo TS from reactant and the more negative reaction energy of endo product from the reactants. This could possibly be explained by the Secondary Orbital Interaction occurring exclusively in endo transition state. For the endo TS, secondary orbital interaction occurs on the overlap of positive phases between the p-orbitals of newly-formed pi system on diene and empty p-orbital of two oxygen atoms, while the strong primary interaction still occurs on both endo and exo state. As a result, endo state has become a both thermodynamically and kinetically stable product.
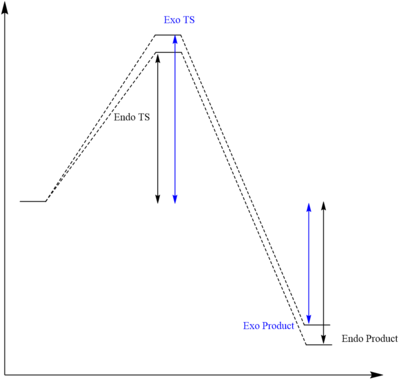
Nf710 (talk) 10:50, 17 November 2017 (UTC) You are contridicting your results. You have predicted that the exo is the thermodynamic product. You have understood the SOO though. You have unfortunatlymiss calculated up your energies. hence why your make is so low for this question. You should make sure that you use the sum of electronic and thermal free energies. this will give your energies at room temperature.
Calculations
File:Exo ts 631 scc215.logFile:Endo 631 scc215.logFile:Exo product scc215.logFile:13 dioxole scc215.logFile:Diene 631 scc215.logFile:Endo ts 631 scc215.log
Exercise 3: Exercise 3: Diels-Alder vs Cheletropic

Optimized TS with corresponding frequencies and Product via two pathways
| Pathway | Vibration | Shape of product |
|---|---|---|
| Endo Diels-Alder |  |
 |
| Exo Diels-Alder |  |
 |
| Cheletropic |  |
 |
(Be careful - you've use the endo product twice Tam10 (talk) 16:37, 12 November 2017 (UTC))
IRC calculated for each possible pathway
As shown in the IRC animation in Table 5, the C-S and C-O bond formation in Diels-Alder reactions are asynchronous while the formation of two C-S bonds are synchronous. It is possibly believed to be related to the difference of van der waals radius in oxygen and sulphur respectively. Sulphur has a larger radius such that the C-S is easier to form than C-O bond.
| Pathway | Animation of IRC |
|---|---|
| Endo Diels-Alder |  |
| Exo Diels-Alder |  |
| Cheletropic |  |
Energy Profile and Energies
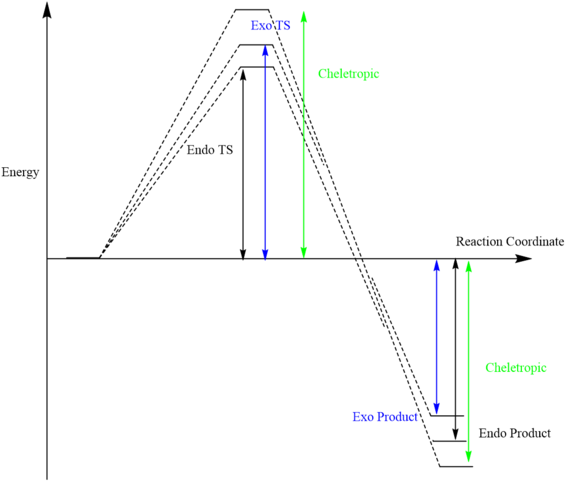
Reaction profiles are constructed using the calculated energy extracted from IRC log files. Since zero was set to be the energy level when the reactant have infinite separation (i.e. reactant energy level = 0 Hatrees), table 6 is showing the corrected relative energy with reference to the zero set.
| Corrected Energies (Hatrees/molecule) | Reactants | TS | Product | ||
|---|---|---|---|---|---|
| Endo Diels-Alder | 0 |
|
| ||
| Exo Diels-Alder | 0 |
|
| ||
| Cheletropic | 0 |
|
|
(I can't reproduce your numbers. Your activation energies are too low and reaction energies too high. Also you show the endo DA as being the more thermodynamically favourable but in your table it is the exo DA Tam10 (talk) 16:37, 12 November 2017 (UTC))
Calculated Reaction energies and Reaction Barrier
Endo Diels-Alder Reaction
Reaction Energy:-0.06415*4.3597439422E-18/1000*6.02E-23 = -168.42715058 kJ/mol
Activation Energy:0.00764995*4.3597439422E-18/1000*6.02E-23 = 20.084945255 kJ/mol
Exo Diels-Alder Reaction
Reaction Energy: -0.06559*4.3597439422E-18/1000*6.02E-23 = -172.1934306 kJ/mol
Activation Energy: 0.00955814*4.3597439422E-18/1000*6.02E-23 = 25.094898482 kJ/mol
Cheletropic Reaction
Reaction Energy:-0.08815 *4.3597439422E-18/1000*6.02E-23 = -231.45070758 kJ/mol
Activation Energy: 0.01739184*4.3597439422E-18/1000*6.02E-23 = 45.662279398 kJ/mol
Base on the calculations made above, the endo D-A reaction is found to be the most kinetically stable reaction, while the cheletropic reaction is the most thermodynamically favoured reaction with a reaction energy as negative as -231.45070758 kJ/mol yet with the highest energy barrier to reach the TS. This is due to the synchronous bond formation would reduce the O-S-O bond angle such that they would experience unfavourable electron-electron repulsion. On the other hand, a 5-member heterocycle formation will lead to a less strained ring than a hydrocarbon cyclic structure since the van der waals radius of S is larger than C atom, such that the ring formation becomes more stable.
Unstable o-xylylene molecule and second possible site of D-A addition
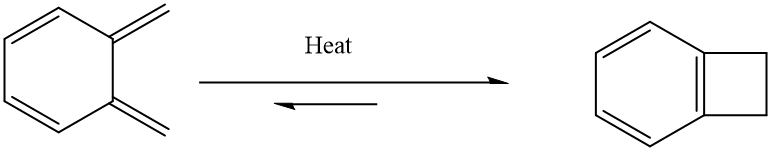
As shown in Fig. 12, there is a possibility for xylylene molecule to undergo a 4-pi electrocyclic reaction to form benzocyclobutene especially in the presence of heat.[3] This is due to the 6 member ring will turn into a 6-pi delocalised electron system after any reaction take place in the diene position, such that the product is more stabilized and favoured to be formed rather than staying as o-xylylene form.
(Did you try to run these calculations? Tam10 (talk) 16:37, 12 November 2017 (UTC))
Calculations
File:CHELETROPIC TS scc215.LOGFile:EXO TS2 scc215.LOGFile:ENDO TS scc215.LOGFile:Final endo irc scc215.logFile:Final exo irc scc215.logFile:Final cheletropic scc215.log
Conclusion
The experiment has given a brief introduction to use computational environment for simulation of reactions and analysis of PES. It has shown several pros and cons by such method.
Although the computational simulation has been more efficient and accurate than traditional measurement and bench work method to predict and analyze a reaction, like simulate the energy level and structure of TS during a reaction which might be impossible to isolate the reaction, the method still has a strong limitation is that it does not take the practicability of reaction in to account. Take the Diels-Alder reaction in exercise 1 for example, although Gaussian could run a simulation on the reaction given that the orbitals and conformation are correct, it still neglected the presence of heat during the reaction. Hence, it is generally believed that butadiene is a poor diene and ethylene is a poor dienophile with wide energy gap to cause a poor overlap for the formation of product.It is also believed that the butadiene will adopt a trans-conformation to minimize the steric clash such that the reaction is impractical in reality.
Reference
1.D. Lide, Tetrahedron, 1962, 17, 125-134.
2.A. Bondi, J. Phys. Chem., 1964, 68, 441–451.
3. Mehta, G.; Kotha, S., Tetrahedron Lett., 2001,57.
