Rep:Mod:treason3
Part i: Investigation into Borane
Optimisation


Borane BH3 was initially using optimised using Gaussian. The final result is displayed below in jmol format. This optimisation will give an approximation closest to borane in the gas phase.
borane |
From the summary file it was found that the calculation type was FOPT, and the method employed was RB3LYP with a 3-21G basis set. The energy of the molecule was calculated as -26.46 Hartrees which is equivalent to -69470 kj.mol-1. The RMS gradient is 0.00020672 Hartree/Bohr. The recorded dipole moment is zero debyes which is to be expected as the molecule is entirely symmetric. The point group recorded is D3H. This is in line with the fact that there is a c3 axis of symmetry through the boron atom and a plane of symmetry perpendicular to this axis.
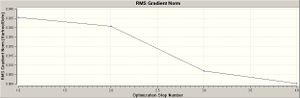
The borane molecule is minimised as described by the graphs to the right hand side. As the bond length is reduced the energy of the molecule decreases until it has reached a minimum. This minimum happens to be global in the case of optimising borane, however its often a local minimum that is computed prompting further calculations to find the global minimum. Gaussian works by minimising the gradient of the potential energy surface. The gradient is defined as the rate of change of the dependent variable (potential energy in this case) with respect to the independent variables (bond lengths, bond angles etc.). Minimising the RMS gradient is equivalent to minimising the forces acting on the molecule. As demonstrated below:
F=-dV/dx {with F being force and V being potential energy}
since the gradient is quoted as the root-mean-square, it becomes a small positive number. An ideal energetically minimised molecule would be one in which the intramolecular forces are equal to zero.
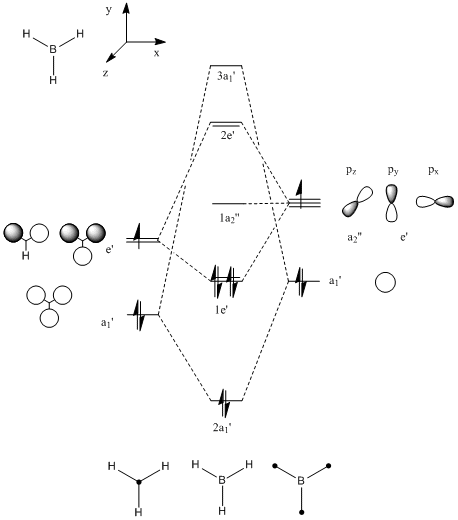
Computational Molecular orbitals and the LCAO approach

https://www.ch.imperial.ac.uk/wiki/images/5/5b/TOMFULLER_BH3_MO.LOG
There is good correlation between the linear combination of atomic orbitals and the filled (bonding) molecular orbitals. The correlation between the empty MOs and the LCAOs is slightly less although still reasonable with no significant differences. The calculated molecular orbitals are known to be more diffuse for empty orbitals and therefore will have a greater deviation from the LCAO description.
Overall this demonstrates that qualatitive MO theory is an adequate enough representation of the electron density of a simple molecule with a high degree of symmetry to predict reactivity amongst other things. For instance a nucleophile will add in to the non-bonding MO in either above or below the plane of the molecule c.f. hydroboration.
Natural Bond Analysis
The optimised BH3 bond distance was found to be 1.19A for all bonds, with a 120 degree angle between the bonds. The orbital summary gives B-H bond occupancy of 1.99853 with an energy of -0.44 Hartrees. The lone pair on Boron has an occupancy of zero with an energy of 0.67 Hartrees, reflecting boranes electron deficient nature. The bonding is sp2 hybridised.
The charges located on the molecule are described both qualitatively and quantitively below. With 1 negative charge unit being equivalent to an electron.
 |
 |
Part ii: Examining the Molecular Orbitals of Diborane
Of further interest is a study into the bonding of diborane, the dimer was initially optimised using a 3-21G basis set in the same way as for borane. The energies associated with the molecule were then calculated also as before using the 6-31G basis set. As expected diborane was found to be lower in energy to the equivalent energy of two borane molecules by 0.34 hartrees/particle. The reason for this is predicted as being the stabilising effect from the 3 centre 2 electron bond formed in B-H-B. The significant molecular orbitals in bringing this about were the homo-1, the homo-3 and to a lesser extent the homo-5. These are described to the right. They demonstrate significant enough electron density located between the B-H-B bonds for it to be plausible to assign hydrogen divalency.
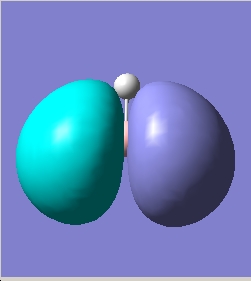 |
 |
 |
The bridging hydrogens were found to have bond lengths slightly longer than the monovalent hydrogens 1.32A as opposed to 1.19A. The reason for this is attributed to the fact there is slightly less electron density located between the Boron atom and the bridging Hydrogens ( 1.5 electrons as opposed to 2 electrons). And therefore it is a slightly weaker bond.
Part iii: Vibrational Analysis of Boron trichloride

Frequency analysis is necessary in order to obtain a theoretical prediction of the IR absorption spectrum. It serves as a test to find if we have reached the minimised energy structure, if negative frequencies are obtained then the molecule is not at an energetic minimum. By comparing the predicted absorptions with the experimental data it is possible to assess the accuracy of the modelling technique.
https://www.ch.imperial.ac.uk/wiki/index.php/Image:TOM_FULLER_BCL3_FREQ.LOG
Boron trichloride was optimised in the same way as for Borane with Gaussian and a LANL2MB basis set. Again using the RB3LYP method and the LANL2MB Basis set, the infra-red frequencies of absorption relating to the different vibration modes of the molecule was calculated. The optimisation calculation took 8 seconds and the frequency calculation took 11 seconds. The spectrum is displayed to the right hand side. And the vibrational forms are described below in pictorial form. Since we have obtained positive rather than negative frequencies of absorption for all vibrations this implies that we have in fact reached a minimum, as the frequencies are physically realisitic.
Frequency of vibration is related to the bond strength of a molecule via the following equation:
f = (k/ų)1/2 with f as the frequency, k as bond strength and μ as reduced mass of the diatomic molecule.
By calculating the gradient of the potential surface of we are in effect finding the negative force as described previously: F=-dV/dx By invoking Hooke's law: F=-kx therefore: k=d2V/dx2
We find the second derivative of the potential energy surface (PES) is equal to k, the bond strength which is proportional to the square of the frequency. This is why it is necessary to run the optimisation of BCl3 with the same basis set as the frequency analysis as they are essentially related.
There are less than six peaks in the spectrum because vibrational numbers 1 and 2 have the same energy, i.e. they are degenerate vibrational modes. This is also the case for numbers 5 and 6. The result of this is that the frequencies of absorption in the IR for these modes are not distinct. A further reason for there being less peaks is that vibrational mode 4 has zero intensity, this is due to the complete symmetry of the stretching. This means that there is no overall change in dipole moment which is necessary to register an IR absorption.
The optimised BCl3 bond distance was found to be 1.87A with 120° bond angle between B-Cl bonds. This compares well with the literature value of 1.75A.1
Gaussview fails to draw chemical bonds if they do not fall within fixed parameters for bond length. This is unimportant however as 2 dimensional "stick-like" bonds exist merely to guide the eye. A better definition of a chemical bond is an area between atoms that contains localised electron density. Providing a mutual attraction of their respective nuclei.
The symmetry expected for BCl3 in the ground state is D3H Gaussview uses D3H symmetry as well. Once Gaussview has constrained the symmetry, Gaussian calculation program will not alter it. Therefore it is far better to distort the symmetry of a molecule prior to running a Gaussian calculation in order to allow it to find flexibility in finding the energy minimum. This is particularly true in the case of ammonia, which will stay planar during optimisations if Gaussview sets the symmetry as D3H, rather than allowing it invert in umbrella-fashion as ammonia is known to do.
Part iv: Optimisation of Mo Complexes followed by Vibrational Analysis
Geometric optimisation
https://www.ch.imperial.ac.uk/wiki/index.php/Image:TomFuller_Mocisnewgeom_opt.out https://www.ch.imperial.ac.uk/wiki/index.php/Image:Tomfuller_Motransnewgeom_opt.out
The Mo(CO)4(PCl3)2 compounds (both cis and trans) are initially loosely optimised via the B3YLP method and the LANL2MB basis set.This basis set provides a pseudo-potential which models the core electrons as a particular function, negating the need to model their behaviour via a rigorous treatment of the Schrodinger equation. This dramatically cuts down on the computing time for the calculation, whilst providing only a small drop in accuracy for heavy atoms.
A manual distortion was then carried out on the molecule. For the cis isomer the Cl atom on one group was set parallel to the axial carbonyl, and a Cl atom on the other group was set parallel to the other axial carbonyl group. For the trans isomer, the molecule was distorted in such a way as to ensure that a P-Cl bond in each group lay parallel to a carbonyl group, additonally the PCl3 groups were arranged in a staggered conformation.
With theses distortions the molecule was optimised in such a way that the global potential energy minimum, rather than the local minimum was located. The molecule was now optimised via Gaussian with increased electronic convergence, and a more sophisticated basis set and pseudo-potential, namely LANL2-DZ. Whilst this ensured greater accuracy it also increased computational time, therefore these calculations were submitted to the SCAN supercomputer.
cis-Mo(CO)4(PCl3)2:
Mocis_optimised |
trans-Mo(CO)4(PCl3)2:
Motrans_optimised |
Geometry:
| Bond | Trans | Cis |
|---|---|---|
| eq. Mo-C | 2.06A | 2.01A |
| ax. Mo-C | - | 2.06A |
| eq. C=O | 1.17A | 1.18A |
| ax. C=O | - | 1.18A |
| Mo-P | 2.45A | 2.51A |
| P-Cl | 2.24A | 2.24A |
Additionally the equatorial C=O ligands in the cis complex are 87.1' from each other, as opposed to the 90' bond angles this is rationalised by the repulsive effect of the bulky phosphine ligands distorting the carbonylys from their ideal 90' bond angles.
Trans has energy of -623.56 Hartrees/particle, whilst cis is -623.58 Hartrees/particle. This means that cis is more stable by 0.02 Hartrees/particle or 60 kj/mol. Theoretically we would expect the cis isomer to be more thermodynamically stable. The rationalisation of this stability lies in the fact that Phosphorus on the ligand is able to delocalise its lone pair onto the complex. Because in the cis isomer each PCl3 ligand lies opposite a carbonyl ligand the molecule will experience what is referred to as the trans-effect. This means the lewis basic phosphorus is able to delocalise its electrons onto the Metal centre, which are then able to further delocalise onto the carbonyls via donation into the C=O pi* bond. This backbonding will lead to an overall stabilising effect of the molecule. In the trans isomer this PCl3, C=O interaction is not possible as the PCl3 are now opposite to each other.
This backbonding is further reflected in the bond distances with the metal cabonyl being 0.05A shorter on the equatorial carbonyls of the cis complex than the trans. Aditionally the Mo-P bond is 0.06A shorter for the cis, and the equatorial C=O bond 0.01A longer, this is again because of backbonding with electron density being donated into the carbonyl pi* orbital, weakening the bond.
As well as these electronic effects, there is also the issue of sterics if one were to increase the size of the ligand its predicted that the cis isomer will become relatively less stable. For instance cis-Mo(CO)4(PPh3)2 would be predicted as being less stable than Mo(CO)4(PMe3)2. Due to the minimisation of steric repulsions by having the bulky phosphorus ligands trans to each other
Metal complexes with a high degree of backbonding such as the cis species would also be predicted to have a slower rate of reaction via a dissociative mechanism than the trans species. This is because the metal-carbon bond is predicted to be stronger and therefore its more difficult to cleave the carbonyls from the molecule.
Vibrational Analysis
https://www.ch.imperial.ac.uk/wiki/index.php/Image:Mo_cis_new_geom_frequency.out https://www.ch.imperial.ac.uk/wiki/index.php/Image:Mo_trans_new_geom_frequency.out
There are no vibrations with negative frequencies indicating that the molecule is fully optimised. The spectrum is displayed to the right hand side.The cis isomer took 28 minutes 19.5 seconds to calculate, and the trans isomer took 39 minutes 2.8 seconds to calculate.


The frequencies to the lowest end of the spectrum are associated with bending modes of vibration. These vibrations are very low energy and are therefore readily accessible at Room temperature. Where molecules have an associated energy of RT which is approximately equal to 2.5 kj/mol. Thus these vibrations can take place without any external IR source acting on them.
The absorptions of the carbonyl stretches are slightly lower wavenumbers for the cis than the trans isomer. This corresponds to a lower energy absorption and reflects the greater degree of backbonding weakening the C=O bond as previously described.
Cis IR stretches - 1807, 1808, 1818 and 1874 cm-1 from left to right:
 |
 |
 |
 |
Trans IR stretches - 1809, 1809, 1832 and 1882 cm-1 from left to right:
 |
 |
 |
 |
The cis isome has lower symmetry and one would therefore expect more peaks in an IR spectrum, as it is easier to break the symmetry and thus create a changing dipole moment. This is reflected in the results of the calculation, where the trans isomer has only one distinct absorption of high intensity. The absorption at 1882cm-1 has no intensity because its total symmetry results in no changing dipole moment necessary to react with IR radiation.
The calculated spectra are slightly lower than the experimental 2 which has the trans isomer absorbing at 1896 cm-1; and the cis isomer absorbing at 1882, 1903, 1917 and 2022 cm-1.
Part v: Comparasion of Structures of cis and trans platin
Cis-platin is a powerful anti-cancer drug. The compound is first hydrated and then carried to the DNA, where the DNA molecule will coordinate to the metal centre. In this way cancer can be prevented from replicating in the cells. Whilst cis-platin retains this valuable property remarkably trans-platin does not have this effect.3
Initially loose optimisations were carried out on cis-platin, trans-platin, the hydrated version of these and cis-paladium. These optimisations were run with the B3YLP method and the LANL2MB basis set, this was chosen due to its ability to take into account a pseudo-potential, thus suitable for a heavier atom with many inner electrons like platinum. After the initial optimisation a more detailed basis set was employed, LAN2DZ, due to the more extensive calculations required these were run on the SCAN supercomputer overnight.
borane |
Thermodynamic Stability
As we can see the cis-platin adopts a square planar conformation. The energy of the molecule is calculated to be -262.30 Hartrees, which is equivalent to -688660 kj.mol-1. This compares with an energy of the trans conformer of -262.32 Hartrees, which is equivalent to -688720kj.mol-1. This difference gives trans as 60kjmol-1 more thermodynamically stable than cis. A rationalisation of this is that the trans isomer will separate the highly electronegative chlorine atoms to a greater degree minimising the unfavourable electrostatic repulsions that will occur between the two atoms.
The charges arising from the platin complexes are shown below in pictorial form.
 |
 |
Natural Bond Analysis
Natural bond analysis shows a small positive charge arising on the platinum atom for each complex, 0.04 and 0.05 for cis and trans respectively. This is relative to a scale in which the magnitude of charge on one electron is equal to one. From this we can see that platinum will be attractive to incoming nucleophiles. In the case of this drug this nucleophile will often take the form of water.
Shown below are the relative charges residing on the aquaplatin complexes. They are 0.30 and 0.31 for cis and trans respectively. This reflects the more electron deficient nature of the central platinum atom and will lead to it being more susceptible to nucleophilic attack and more easily coordinated to DNA.
 |
 |
Geometry
Whilst the bond angles on the compounds deviate from 90° this is rationalised by the fact that Gaussian computes atoms at fixed coordinates where as in reality the the NH3 will be constantly rotating leading to an averaging process. Therefore all the complexes are assumed to have a square planar arrangement with 90° between bond angles.
| Compound | Pt-Cl | Pt-O | Pt-N |
|---|---|---|---|
| cisplatin | 2.41 | - | 2.11 |
| transplatin | 2.43 | - | 2.07 |
| aqua cisplatin | 2.38 | 2.11 | 2.12 (trans to Cl), 2.05 |
| trans aquaplatin | 2.36 | 2.15 | 2.08 |
The most significant data above is the fact that the exchange of one chlorine ligand with an aqua ligand leads to a reordering of the cis vs tran Pt-Cl bond length. For cisplatin the Pt-Cl bond length is 0.02A shorter than trans platin; however for the aqua complex the cis has the longer Pt-Cl bond strength than trans by 0.02A. this implies that this is a weaker bond in aqua cisplatin and this may provide evidence as to why cis is active in binding to DNA.
Vibrational Analysis
The vibrational spectrum of both the cis and trans complexes were predicted using the same basis set, LANL2DZ. This was necessary because the square of the frequency is proportional to the force constant of the bond and this is in turn equal to the second derivative of the potential energy surface. Therefore in order to be consistent its necessary to stay to the same basis set.
| The IR spectrum of cisplatin: |  |
The IR spectrum of transplatin: |  |
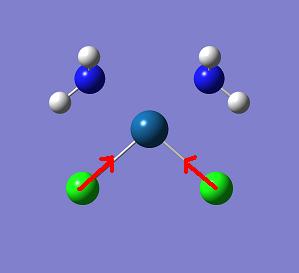
The vibrational modes for the Pt-Cl bond are of particular interest. There are 2 vibrational modes. These exist at 300 and 320cm-1 for trans platin and at 328 and 330cm-1 for cis platin. The symmetric stretch for the trans (320cm-1) and the cis (330cm-1) are displayed pictorially below.
 |
 |
The fact that the Pt-Cl stretch is at lower energy for the trans compound is a reflection of it being a weaker bond than in the cis isomer.
Molecular Orbital Analysis of Transplatin
 |
 |
 |
 |
The MO analysis above shows the weakened Pt-Cl bond. Both the HOMO and the HOMO-1 both have a nodal plane in contained within the Pt-Cl bond. Its known that the HOMO will often determine the character of the bond, therefore its not suprising that the Cl bond is very weak as there is a high degree of antibonding character. This antibonding Pt-Cl character is present again in the LUMO. Suggesting that the ligand exchange necessary for aquation may take place via an associative or possibly interchange mechanism with the water molecule adding in as a nucleophile above or below the plane of the molecule. This addition will simultaneously weaken the Cl bond forcing it to decoordinate.
cisplatin optimisation: https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/to-2817 aqua-cisplatin optimisation: https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/to-2821 aqua-transplatin optimisation: https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/to-2823 transplatin frequency: https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/to-2824 cisplatin frequency: https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/to-2825 transplatin MO: https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/to-2819
Bibliography
1. C. Spencer and W. N. Lipscomb, J. Chem. Phys., 1958, 28, 355
2. A. D. Allen and P. F. Barrett, Can. J. Chem., 1968, 46, 1649
3. S. Nafisi and Z. Norouzi, DNA and cell biology, 2009, 28, 469-477 DOI: 10.1089=dna.2009.0894






