Rep:Mod:tjasdo23klfu10ddj123
Part 1
The Hydrogenation of Cyclopentadiene Dimer
The first part of the assignment involved the use of molecular mechanics techniques to identify the major product of the cyclodimerisation of cyclopentadiene which can form either the endo or exo dimer. Molecular mechanics modelling technique provides a way to optimise the geometry of the dimer and the hydrogenated products on the basis of their energies and hence enables the evaluation of whether the reaction proceeds via kinetic or thermodynamic control.
Endo or exo dimer?
Cyclopentadiene can dimerise to form either the endo dimer or the exo dimer via a pericyclic Diels Alder reaction. This proceeds through a concerted [p4s+p2s] and [p2s+p4s] cycloaddition reaction mechanisms.
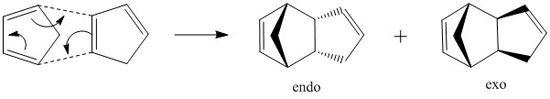
The endo product is specifically formed and using molecular mechanics, this can be seen to be proceed through kinetic control since the endo dimer is higher energy than the exo dimer which corresponds to the thermodynamic product. The energy data was calculated using Avogadro; by optimising the the geomoetry of each configuration using the MMFF94s force field the relative energy breakdown was calculated. This can be seen quantitatively in table 1.
| Endo | Exo | Energy Difference | |
|---|---|---|---|
| Total bond stretching energy | 3.46726 | 3.54452 | -0.07726 |
| Total angle bending energy | 33.19266 | 30.77951 | 2.41315 |
| Total stretch bending energy | -2.08207 | -2.03966 | -0.04241 |
| Total torsional energy | -2.94878 | -2.76245 | -0.18633 |
| Total out-of-plane bending energy | 0.02193 | 0.01461 | 0.00732 |
| Total van der waals energy | 12.35645 | 12.82878 | -0.47233 |
| Total electrostatic energy | 14.18327 | 13.01404 | 1.16923 |
| Total energy ( kcal/mol) | 58.19071 | 55.37936 | 2.81135 |
Analysis of the different energy contributions show that the angle bending energy and the electrostatic energy contributions have greatest effect on the energy difference between the two configurations. Up to a temperature of ~150°C, the endo product is almost the only dimerisation product. This is a direct result of significant attractive secondary orbital interactions in the transition state between the HOMO of one cyclopentadiene with the LUMO of the other, leading to the endo dimer.[1].
In contrast, secondary orbital interactions are completely absent in the exo transition state. This phenomenon combined with steric repulsion in the transition state, results in overall destabilisation of the exo transition state thus making it kinetically unfavourable.[2] However, at higher temperatures the system has sufficient energy to overcome the higher activation energy to the thermodynamic transition state and the energetically more stable exo dimer is formed.
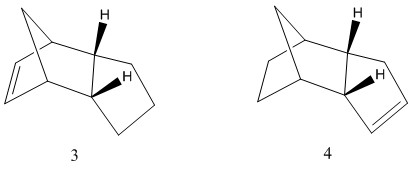
Hydrogenaton of Endo dimer
Like the dimerisation of cyclopentadiene, the hydrogenation of the specifically formed endo product at ambient temperature can occur under kinetic or thermodynamic control. This results in two possible products 3 and 4, and the corresponding energies associate with each product is tabulated in table 2.
| Product 3 | Product 4 | Energy Difference | |
|---|---|---|---|
| Total bond stretching energy | 3.30737 | 2.82339 | 0.48398 |
| Total angle bending energy | 30.85459 | 24.68521 | 6.16938 |
| Total stretch bending energy | -1.9262 | -1.65734 | -0.26886 |
| Total torsional energy | 0.07526 | -0.37915 | 0.45441 |
| Total out-of-plane bending energy | 0.01529 | 0.00028 | 0.01501 |
| Total van der waals energy | 13.27556 | 10.6381 | 2.63756 |
| Total electrostatic energy | 5.12097 | 5.147 | -0.02603 |
| Total energy ( kcal/mol) | 50.72285 | 41.25749 | 9.46536 |
The lower energy of the dihydro derivative product 4 indicates that it is formed initially via the hydrogenation of the norbornene part of the dimer. The hydrogenation of the norbornene double bond occurs five times faster than that of the cyclopentene ring.[3] Furthermore, it is the energetically more stable thermodynamic product as concluded by energy calculation using molecular mechanics technique.
The energy difference between the two configurations are largely due to the angle bending energy and van der waals energy. The angle bending energy is attributed to the bending of bonds away from the equilibrium angle; this effect is much more apparent in product 3 due to the norbornene part of the molecule containing the cyclohexene ring with a methylene bridge which results in significant ring stain thus bending from equilibrium angle. More specfically, the angle strain energy can be attributed to the deviation of the bond angle around the sp2 C centre which in product 3, is 107.2° from the ideal 120° sp2 bond angle. Comparatively, this deviation is lower in product 4, which has 112.5° around the sp2 C centres.
The other major energy contribution is the van der waals energy which is more significant in product 3 as a result of hydrogenation of the cyclopentene double bond, leading to repulsion between adjacent H atoms on the sp3 C atoms. Both products 3 and 4 are lower energy than the dimer precursor indicating the hydrogenation is exothermic.
After prolonged hydrogenation, the tetrahydro derivative is formed.
Atropisomerism is a type of stereoisomerism which gives distinction between two molecules and arises due to the hindered rotation about a single bond caused by the steric strain energy barrier resticting free rotation.[4] This part of the task involves the evaluation of two atropisomers, 9 and 10, to identify which is more stable.
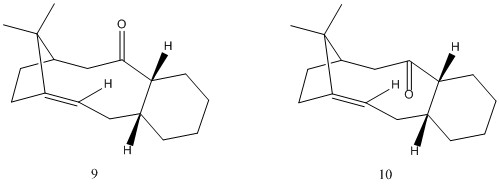
9 and 10 are are 2 possible intermediates in the synthesis of Taxol. Taxol is used in cancer therapy as an anticancer drug. Its anticancer activirt works by inhibiting mitosis through enhancement of the polymerisation of tubulin and consequently renders microtubules more stable.[5]
Using Avogadro, the structures of 9 and 10 were optimised, and the MMFF94s force-field was applied to calculate the relative energies to determine the most stable isomer, illustrated in table 3. Each atropisomer is presented in two distinct conformations of the cyclohexane ring, which exists in 2 distinct conformations, chair and twist-boat. This gives rise to distinct relative energies of the molecule. Atropisomer 10 in the chair conformation is calculated to be the most stable.
| 9 (chair) | 9 (twist-boat) | 10 (chair) | 10 (twist-boat) | |
|---|---|---|---|---|
| Total bond stretching energy | 7.67498 | 7.93462 | 7.66369 | 7.75584 |
| Total angle bending energy | 28.26915 | 30.10254 | 19.11607 | 19.02636 |
| Total stretch bending energy | -0.0764 | -0.03417 | -0.11426 | -0.13197 |
| Total torsional energy | 0.2381 | 3.03923 | 0.0774 | 3.75011 |
| Total out-of-plane bending energy | 0.97356 | 0.95798 | 0.87153 | 0.9492 |
| Total Van der waals energy | 33.15829 | 35.64247 | 33.1741 | 35.00339 |
| Total electrostatic energy | 0.3008 | 0.29727 | -0.0645 | -0.06327 |
| Total energy (kcal/mol) | 70.53849 | 77.93993 | 60.72403 | 66.28965 |
Isomer 9 is first formed via its carbinol precursor via an atropselective anionic oxy-Cope rearrangement. The syn orientation of the two stereogenic cyclohexnyl protons relative to the gem-dimethyl substituted bridge requires that the rearrangement proceed through an endo chair transition state to first form the enolate. The enolate then undergoes a strongly thermodynamically favoured tautomerisation to the carbonyl, which essentially renders the oxy-Cope rearrangement irreversible. The rearragement proceeds through the endo chair transition state results in the consequently formed carbonyl group to be orientated in an upward direction proximal to the apical dimethyl bridge. Thus the initial product formed is the least stable atropisomer.[6][7]
On standing, isomer 9 undergoes bond rotation to form the thermodynamically most stable atropisomer 10 in the chair conformation. The extra stability of isomer 10 (chair) is a result of the molecule having much lower angle bending energy than 9. This can be attributed to the fact that in the intermediate 10, the O atom is orientated away from the cyclohexane ring as well as being on the other side relative to the gem-dimethyl substituted bridge, thus there is lower repulsion of the carbon and hydrogen bonds, resulting in lower bending of the bonds from equilibrium angle.
In contrast, in isomer 9, the O atom is orientated so it is closer to the cyclohexane ring, as well as it being on the same side as the gem-dimethyl substituted bridge. This contributes the largest to the angle bending energy difference between the 2 atropisomers and leads to greater destabilisation of 9.
Stability of bridgehead alkene
The bridgehead alkene double bond reacts particularly slowly as a result of the alkene being less strained relative to the parent alkane. The taxol intermediates which show this property can be terms 'hyperstable' olefins and they have negative olefin strain values. This extra stability of the intermediate can be attributed to the cage structure of the bridgehead olefin. [8]
This part of the assignment involves the use of spectroscopic stimulation to predict the 1H and 13C NMR of one of the intermediate atropisomers in the synthesis of Taxol. The result of the computer simulation is then compared to literature to evaluate whether the simulation correctly interprets and assigns the correct NMR spectrum based on the optimised structure of the intermediate (as carried out in the previous step).
The spectroscopic stimulation was run using the HPC and the molecule used was the chair conformation of atropisomer 18. As calculated from the previous step, this is the thermodynamically most stable conformation of the intermediate which is related to an intermediate in the synthesis of Taxol, as per the previous step.



The calculations of the spectroscopic stimulation is shown in table 4.
| 1H NMR data for Chair conformation of 18 | 13C NMR data for Chair conformation of 18 | |||||
|---|---|---|---|---|---|---|
| Experimental data | Literature data[7] | Experimental data | Literature data[7] | |||
| Chemical shift ppm | Relative integration | Chemical shift / ppm | Relative integration | Chemical shift / ppm | Chemical shift / ppm | Difference / ppm |
| 5.97 | 1 | 5.21 (m) | 1 | 211.92 | 211.49 | -0.43 |
| 3.12 | 2 | 3.00 -2.70 (m) | 6 | 147.87 | 148.72 | 0.85 |
| 2.95 | 2 | 2.70 - 2.35 (m) | 4 | 120.13 | 120.9 | 0.77 |
| 2.89 | 1 | 2.20 - 1.70 (m) | 6 | 92.84 | 74.61 | 18.23 |
| 2.80 | 2 | 1.58 (t) | 1 | 65.94 | 60.53 | -5.41 |
| 2.67 | 1 | 1.50 - 1.20 (m) | 3 | 54.93 | 51.3 | -3.63 |
| 2.54 | 2 | 1.1 (s) | 3 | 54.76 | 50.94 | -3.82 |
| 2.43 | 1 | 1.07 (s) | 3 | 49.53 | 45.53 | -4.00 |
| 2.31 | 2 | 1.03 (s) | 3 | 48.04 | 43.28 | -4.76 |
| 1.98 | 2 | 45.65 | 40.82 | -4.83 | ||
| 1.83 | 2 | 44.00 | 38.73 | -5.27 | ||
| 1.64 | 1 | 41.47 | 36.78 | -4.69 | ||
| 1.53 | 3 | 38.51 | 35.47 | -3.04 | ||
| 1.34 | 1 | 33.69 | 30.84 | -2.85 | ||
| 1.27 | 1 | 32.47 | 30 | -2.47 | ||
| 1.21 | 2 | 28.36 | 25.56 | -2.80 | ||
| 0.96 | 2 | 26.50 | 25.35 | -1.15 | ||
| 0.90 | 1 | 24.45 | 22.21 | -2.24 | ||
| 0.60 | 1 | 24.01 | 21.39 | -2.62 | ||
| 22.58 | 19.83 | -2.75 | ||||
The results show relatively good match for 13C NMR, whereas for 1H NMR, the experimental data shows some discrepancies to the literature data. The slight mismatch between the in the experimental 1H NMR data can be attributed to the fact that using the HPC procedure, the NMR simulation is done using a static molecule. This results in an incomplete analysis of molecule because bond vibrations and rapid bond spinning is neglected in the calculation. A direct consequence of this problem is that the experimental data shows 19 distinct proton environments (compared to the 9 reported in the literature) due to the lack of bond rotation considered in the computer simulation. If the experimental 1H NMR was computed using a more realistic molecule, some of the calculated peaks can be expected to be degenerate, thus combining to form one peak of higher integration. Moreover, the literature seems to have grouped several peaks into a chemical shift range and reported them as multiplet H environments. These two observations combined, may explain why the 1H NMR calculation shows deviation from literature.
The 13C NMR data by and large shows good agreement with the literature. The same number of carbon environments are calculated as that reported in the literature, however most of the experimental chemical shift values are calculated to be slightly more deshielded than literature, thus a higher chemical shift value. There is also a peak reported in the experimental data at 92.84 ppm which is anomalously high relative to the other deviations. This C environment corresponds to the C atom attached to two S atoms. This anomalously large chemical shift difference and other smaller deviations from literature is likely to be a result of slight differences in the conformation of the literature molecule compared to the conformation used in this calculation, leading to slightly difference chemical environments, thus giving rise to small deviations in chemical shift.
Gibbs free energy of the atropisomers
The gibbs free energies corresponding to the two isomeric configurations of the molecule are shown below.
| Molecule | Gibbs free energy / Hartrees |
|---|---|
| 17 | -1651.440112 |
| 18 | -1651.463243 |
| Energy difference | 0.023131 |
1 Hartree = 2625.5 kJ/mol
The difference in gibbs free energy = 0.023131 hartrees = 60.73 kJ/mol
The gibbs free energy is given by the sum of the electronic can thermal free energies. The negative gibbs free energy indicates that both isomers are in their stable state relative to their molecular precursor. Furthermore, the small difference in free energy between the isomers suggests their is very little change in structure between them. The ground state energy of 18 is much lower than that of 17 which is expected from the experimental observations discussed previously.
Part 2
The two catalytic systems
In the 1S Synthesis experiment, 4 alkenes were epoxidised using two different catalytic systems using the Shi asymmetric Fructose catalyst and the Jacobsen asymmetric catalyst. The experiment was done as a pair, where each individual epoxidised two of the four given alkenes, using both the shi catalyst and jacbosen catalyst. The products of the experiment were 4 epoxides of unknown absolute configurations in an enantiomeric excess.
Shi asymmetric Fructose catalyst
Shi epoxidation makes use of the 'Shi catalyst' which is derived from the reaction of D-fructose and oxone. [9] The catalyst is able to converts alkenes into epoxides containing 2 stereocentres. The method of epoxidation using the shi catalyst involves first forming the dioxirane which is generated insitu from the precursor ketone and an oxidising agent; PCC is used in this case. The dioxirane formed then transfers an O to the alkene to form the resulting epoxide.
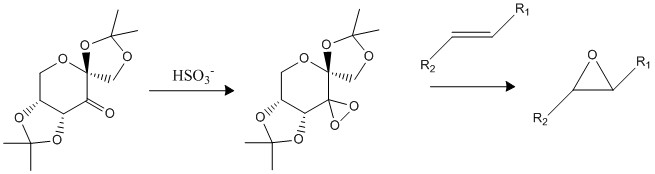
Using the Cambridge crystal database (CCDC) - Conquest program, the precatalyst 21 was found and analysed using the program Mercury. From first look, there are three O-C-O substructures, therefore suggesting 3 anomeric centres are present. However, using molecular mechanics modelling to analyse the C-O bond lengths, it is clear that this is not the case.
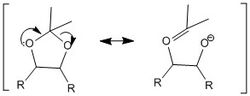
The anomeric effect is a stereoelectronic stabilising effect, whereby the anomeric oxygen atom in a cyclic ring occupies the axial positon so that the O lone pair adopts an anti-periplanar (app) relative to the best acceptor C-O bond. This interaction involves the donation of the oxygen electron lone pair into the s* C-O orbital, leading to an overall stabilising interaction. This interaction results in the lengthening of one C-O bond and the shortening of another and can be illustrated as a no bond-double bond resonance effect.[10] This lengthening of one C-O bond and shortening of the other in a O-C-O substructure is characteristic of the anomeric effect.
Using molecule mechanics modelling, the C-O bond lengths was calculated. Table 5 shows the C-O bond lengths of the three O-C-O substructures within the Shi catalyst.
| C-O bond lengths of Shi pre-catalyst | ||
|---|---|---|
| CO bond length / Å | Bond | Substructure |
| 1.441 | O2 - C7 | O2 - C7 - O1 |
| 1.413 | O1 - C7 | |
| 1.403 | O6 - C2 | O6 - C2 - O2 |
| 1.403 | O2 - C2 | |
| 1.409 | O5 - C10 | O5 - C10 - O4 |
| 1.439 | O4 - C10 | |
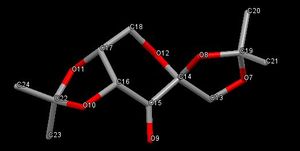
The present of an O-C-O substructure in the structure of the Shi pre catalyst 21, suggests the presence on an anomeric centre. However, from further study of the actual bond lengths using Mercury and the results are interesting. The C-O bond lengths corresponding to the structure shows the presence of three O-C-O substructures, but specifically two of these substructures contain one longer and one shorter C-O bond and the other substructure contains a a set of 2 C-O bonds which are the same length. This implies that only 2 of the 3 O-C-O groups present are actually anomeric centres, whereas the other is not.
Furthermore, this can be shown pictorially in the molecule drawing. The O-C-O substructure protruding from the cyclohexane hetero ring, O6 - C2 - O2, is in fact not anomeric. This is because the oxygen lone pair orbital is orientated in a slightly difference direction relative to the s* C-O orbital, thus good orbital overlap is not possible.
Jacobsen asymmetric catalyst
Jacobsen's catalyst is a coordination compound of manganese and a salen-type ligand. It acts as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform achiral alkenes into epoxides containing chiral centres.[11] Jacobsen's catalyst works specifically well for the epoxidation of cis alkenes and this will be further explored later on. The Mn(III) complex formed in the synthesis of the catalyst, 23, is the pre-catalyst and is activated by bleach (NaOCl)to form the active catalyst species, 24, which epoxdises alkenes in higher selectivity than any other reported synthetic catalysts.[12]
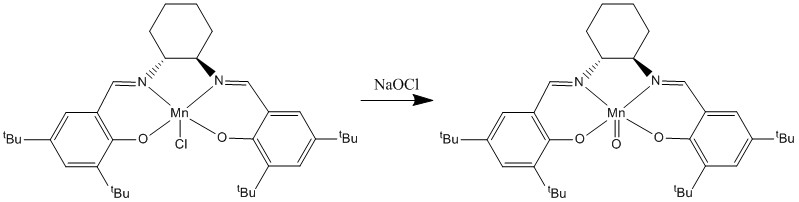


Using CDCC program, following the same process as used for the Shi catalyst, Jacobsen catalsyt was found by searching in Conquest and the resulting query was analysed using Mercury. Using Mercury, the close approach of the t-butyl groups on the adjacent rings in the structure of the pre-catalyst was studied.
Carbon has a Van der Waals' (VdW) radius of 1.7 Å, thus for the interaction of the t-butyl groups the distance mathematically be in the VdW distance of 3-4Å. The program was calculate any interactions between C atoms in a maximum VdW radius sum of 4 Å. The calculation was also limited to interactions at least 5 bonds away to restrict the outcome. Using these settings, yielded one specific contact within the structure corresponding to an interaction of the t-butyl groups of the benzene rings in the catalyst strucure. This interactions occurs between C atoms C59 and C71 and has a distance of 3.697 Å. Furthermore, 3.4 Å is the sum of the VdW radii for two C atoms, therefore a separation of <3.4 Å would indicate a repulsive interaction and a distance slightly larger than 3.4 Å would give an attractive interaction. Since the calculated VdW distance between the t-butyl groups is 3.697 Å, the t-butyl groups are slighty attractive.
When the setting is changed to calculate interactions with a maximum VdW radius of 6 Å, two further interactions between t-butyl groups are found. These have a distance separation of 3.944Å and 3.999Å. These distances are notably larger than 3.4Å, thus at these distances little to no interaction is present.
The presenece of the t-butyl groups renders the catalyst more suitable for the epoxidation of cis alkenes rather than trans. The cis alkene approaches over the diimine bridge of the activated catalyst as a result of the steric hindrance around the Mn centre preventing side-on approach of the alkene, rendering the reaction enantioselective and selective for cis alkenes and not trans. This is enforced by the t-butyl groups in the ortho and para positions to the salen oxygens. [12]
NMR of Styrene Oxide

Styrene is one of the alkenes used in the computational of the epoxidation using the Shi and Jacobsen catalysts. It is a terminal alkene and when epoxidised asymmetrically, one chiral centre is formed in the resulting epoxide. This centre can either be (R) or (S) centered, however in the computational analysis of the epoxide, the (R) form was used.
1H NMR
When compared to literature, the computed 1H NMR values ( in CDCl3) using quantum mechanics are similar in terms of chemical shift values which suggests that the computed conformation of styrene oxide is a realistic one, resembling the real molecule. However, although chemical shift values are relatively similar, the computed NMR calculates 2 distinct H environments at 7.49 ppm and 7.3 ppm, whereas literature reports 5 equivalent H environments giving rise to one peak this region. This peak corresponds to the phenyl ring protons. The discrepancy is a shortcoming of quantum mechanical NMR calculations because it models the molecule statically which neglects the possibility of bond rotation, ring spinning of the phenyl group and migration shifts which renders all five ring protons equivalent - which is not the case in when the molecule is static, as modelled computationally.

| 1H NMR of (R)-Styrene Oxide | |
|---|---|
| Computational | Literature [13] |
| Chemical shift / ppm | Chemical shift / ppm |
| 7.49 (4H) | 7.40 - 7.23 (5H) |
| 7.3 (1H) | |
| 3.66 (1H) | 3.86 (1H) |
| 3.12 (1H) | 3.15 (1H) |
| 2.53 (1H) | 2.80 (1H) |
13C NMR
The Carbon NMR ( in CDCl3) shows relatively good agreement with literature. However, 7 peaks are reported for 7 different C environments whereas the literature shows 6 distinct environments.

| 13C NMR of (R)-Styrene Oxide | |
|---|---|
| Computational | Literature [14] |
| Chemical shift / ppm | Chemical shift / ppm |
| 135.14 (1C) | 137.5 (1C) |
| 124.14 (1C) | 128.2 (2C) |
| 123.41 (1C) | |
| 122.95 (2C) | 128 (1C) |
| 118.27 (1C) | 125.3 (2C) |
| 54.06 1C) | 51.1 (1C) |
| 53.47 (1C) | 51 (1C) |
NMR of Trans-Stilbene Oxide
Trans-Stilbene can be epoxidised to form 2 possible enantiomers: (R,R)and (S,S). As with Styrene Oxide, Computated NMR using quantum mechanical optimisation of the conformation of the structure of the molecule shows a relatively good comparison to that reported in literature.

1H NMR
| 1H NMR of Trans-stilbene oxide | |
|---|---|
| Computational | Literature [14] |
| Chemical shift / ppm | Chemical shift / ppm |
| 7.57 (2H) | 7.25-2.59 (10H) |
| 7.48 (8H) | |
| 3.53 (2H) | 3.87 (2H) |
13C NMR

| 13C NMR of Trans-stilbene oxide | |
|---|---|
| Computational | Literature [14] |
| Chemical shift / ppm | Chemical shift / ppm |
| 134.09 (2C) | 137.1 (2C) |
| 124.22 (2C) | 128.5 (4C) |
| 123.52 (2C) | |
| 123.21 (2C) | 128.3 (2C) |
| 123.08 (2C) | 125.5 (4H) |
| 118.26 (2C) | |
| 66.42 (2C) | 62.8 (1C) |
Absolute configuration of the epoxide products
NMR spectroscopy is a good spectroscopic technique to show that the epoxides were made because the spectra can differentiate between different diastereoisomers. However, it fails to identify the formation of different enantiomeric products because enantiomers show little to no change in the 1H and 13C NMR spectra. The stereochemistry of the chiral centres in the epoxide products can be evaluated using computational optical rotation calculations. The result of this is shown below.
| Optical Rotation ( in CHCl3 at λ=589nm) | ||
|---|---|---|
| Product | Computed value | Literature value |
| Trans-stilbene oxide | 297.91 | 250.8[15] |
| Styrene oxide | 30.27 | 32.1[16] |
The experimental values shows good resemblance to the literature values which to some extent shows that the experimental conformations with the actual conformations that the products exist in in reality.
Transition state analysis for Shi epoxidation of trans-ß-methyl styrene
The epoxidation of trans-ß-methyl styrene using the asymmetric shi catalyst can proceed via 8 possible transition states, each with a distinct energy. The specific transition state through which the kinetically controlled epoxidation proceeds depends on 3 factors: whether the (R,R) or (S,S) epoxide is being formed, which of the dioxirane O atoms are used in the transfer and whether the phenyl group is orientated exo or endo relative to the fructose. Taking all this into account, the transition state which the reaction proceeds through to form the major product has the lowest energy.
In light of this, the major enantiomeric product and enantiomeric excess of the epoxidation can be found by the analysis of the relative energies of the transition states. The energies are shown in the tables below. The relative total energy in each case is taken relative to the lowest energy transition state for the given configuration (highlighted).
| (R,R) - trans-ß-methyl styrene | |||
|---|---|---|---|
| Transition state | Energy / Ha | Relative Total Energy / Ha | Relative Total Energy / kJ/mol |
| O2 endo | -1343.02297 | 0.009473 | 24.8713615 |
| O2 exo | -1343.019233 | 0.01321 | 34.682855 |
| O1 exo | -1343.029272 | 0.003171 | 8.3254605 |
| O1 endo | -1343.032443 | 0 | 0 |
| (S,S) - trans-ß-methyl styrene | |||
|---|---|---|---|
| Transition state | Energy / Ha | Relative Total Energy / Ha | Relative Total Energy / kJ/mol |
| O2 endo | -1343.015603 | 0.009139 | 23.9944445 |
| O2 exo | -1343.017942 | 0.0068 | 17.8534 |
| O1 exo | -1343.024742 | 0 | 0 |
| O1 endo | -1343.023766 | 0.000976 | 2.562488 |

The difference between the lowest energy transition states of each enantiomeric product (R,R) and (S,S) = 0.007701 Ha.

This difference is equivalent to 20.219 kJ/mol.
Therefore, the (R,R) configuration of trans-ß-methyl styrene oxide proceeds through the lowest energy transition state and is the major product.
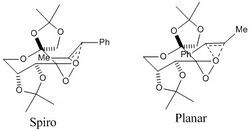
Computational studies show that the epoxidation of alkenes by the Shi catalyst can occur via either a Spiro or a Planar arrangement of the alkene relative to the dioxirane of the activated shi catalyst. The catalyst contains two diastereomeric oxygens which can both transfer to the alkene in the epoxidation, however, the equatorial O (labelled 1 below) is sterically more hindered. This results in one of the O being preferred in the O transfer as the transition state is less destabilised by the steric repulsion from the approach of the alkene. The epoxidation of trans-ß-methyl styrene is most likely to proceed by the abstraction of the least hindered axial dioxirane O and this can proceed through 8 possible transition states of which 4 are spiro and 4 are planar.
The most stable spiro and planar transition states are shown; in these states the groups on the alkene are orientated away from ketal ring A thus reducing steric repulsion in the transition state. The epoxidation proceeds through the lowest energy spiro transition state which gains further stability by the enhanced stabilising secondary orbital interactions between the π* orbital of the alkene and the oxygen lone pair on in the interacting O of the dioxirane.
Through the spiro transition state, the (R,R)-epoxide is formed over the (S,S)-epoxide which is formed via the planar transiton state. Furthermore, the stereoisomer cis-ß-methyl styrene is epoxidised with poor enantioselectivity using the Shi catalyst because in the the 2 lowest energy competing spiro transition states, there is little differentiation between the Ph and Me groups. This results in similar transition state energies. [17]
The computed value of dG = -20.219 kJ/mol.
The enantiomeric excess can be calculated by conversion of the gibbs free energy, dG = -RTlnK to give K by K = exp(-dG/RT).
K is calculated to be K = 3591.
Using ee = (K-1)/(K+1) , ee = 99.9%
This can be compared to literature which gives a value of 95%. [17]
Transition state analysis for Jacobsen epoxidation of cis-ß-methyl styrene
The Jacobsen epoxidation of cis-ß-methyl styrene can proceed through 4 possible transition states which depends on whether the (R,S) or (S,R) configuration of the epoxide is being formed. Depending on which epoxide is being formed, the O atom of the Mn=O substructure can transfer to the alkene in either an endo or exo type arrangement of the alkene relative to the catalyst. The energy of the possible transition states are computed in a similar way to the Shi catalyst and the resulting energies is shown below.
| (R,S) - cis-ß-methyl styrene | |||
|---|---|---|---|
| Transition state | Energy / Ha | Relative Total Energy / Ha | Relative Total Energy / kJ/mol |
| endo | -3383.25106 | 0 | 0 |
| exo | -3383.25027 | 0.00079 | 2.074145 |
| (S,R) - cis-ß-methyl styrene | |||
|---|---|---|---|
| Transition state | Energy / Ha | Relative Total Energy / Ha | Relative Total Energy / kJ/mol |
| endo | -3383.259559 | 0 | 0 |
| exo | -3383.253442 | 0.006117 | 16.0601835 |
The difference between the lowest energy transition states of each enantiomeric product (R,S) and (S,R) = 0.008499 Ha.
This difference is equivalent to 22.31412 kJ/mol.
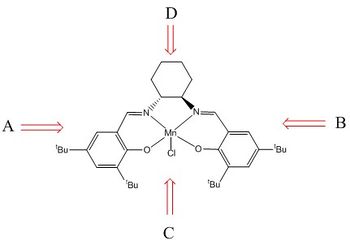
Therefore, the (S,R) configuration of cis-ß-methyl styrene oxide proceeds through the lowest energy endo type transition state and is the major product. This is confirmed in the literature. [12]
The alkene approaches the metal-oxo bond in a side-on perpendicular manner, this is show pictorially to the right. The alkene can approach in four possible directions, however, in this case where the salen ligand contains t-butyl groups, invoking steric hindrance in 3 of these directions (A,B,C) only the one position (D) is possible. Therefore, the alkene approaches the metal-oxo bond over the diimine bridge. Furthermore, on approach towards the O atom, the alkene directs is substituents away from the axial hydrogens of the cyclohexane, resulting in the formation of the (S,R)-epoxide via the lower energy transition state.
The computed value of dG = -22.31412 kJ/mol.
The enantiomeric excess can be calculated by conversion of the gibbs free energy, dG = -RTlnK to give K by K = exp(-dG/RT).
K is calculated to be K = 8155.5.
Using ee = (K-1)/(K+1) , ee = 99.96%
This can be compared to literature which gives a value of 92%. [12]
Non covalent interactions in the active-site of the reaction transition state
NCI analysis was conducted on the lowest energy transition state in the formation of (R,R)-trans-ß-methyl styrene oxide. Non-covalent interactions (NCI) are as the name suggests interactions that do not involve the the sharing of a pair of electrons between two atoms for form a strong bond. NCI interactions include electrostatic attractions, hydrogen bonds and dispersion-like close approaches of pairs of atoms.
The result of the NCI analysis conducted is shown below. Areas of blue show very attractive interactions, green shows mildly attractive, yellow shows midly repuslive and red is strongly repulsive.
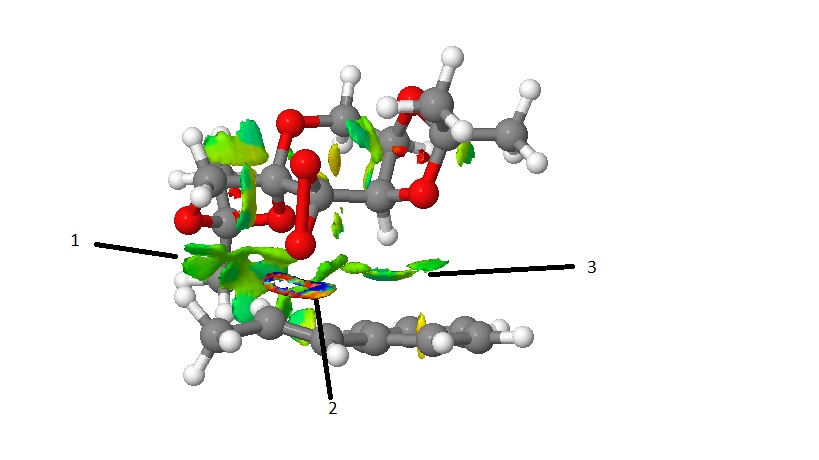
Interaction 1 shown, shows strong Van der Waals' attraction between 2 H atoms of alkene and the 5 membered cyclic ketal ring on the catalyst Label 2 shows the formation of the formaton of the C-O bond of the forming epoxide and the interaction can be considered to be half-covalent. This interaction is normally ignored in NCI analysis. Label 3 indicates the strongly attractive Van der Waals' interaction between a H atom on the catalyst and the C atoms in the phenyl ring of the alkene. It can be seen that in the coming together of the two molecules, most of the interactions are attractive. This is what brings the molecules together and keeps the molecules together so the O of the dioxirane is close enough to undergo transfer to form the resulting epoxide.
Electronic topology (QTAIM) in the active-site of the reaction transition state
Electronic topology analysis of the active-site was also ran for the lowest energy transition state leading to trans-ß-methyl styrene. NCI analysis focuses on non covalent interactions between the catalyst molecule and the alkene whereas QTAIM focuses on the electron density in the covalent regions of the molecule . In these region, there is much stronger interactions compared to the non-covalent regions shown in the NCI analysis.
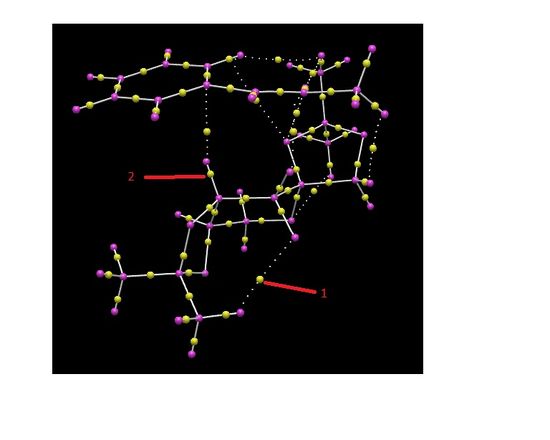
Label 2 indicates a bond critical point (BCP). At this point the derivative of the electron density with respect to each of the three coordinates is zero. This means that at this point there is negligible change in the electron density in the covalent bond region. In contrast, label 1 identifies a BCP of weak non-covalent interaction between an O and a H atom. This weaker non-covalent interaction is also found in the NCI analysis.
Suggested new candidates for investigations
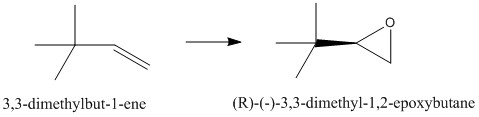
3,3-dimethylbut-1-ene may be a good alkene for further investigation. Epoxidation of the alkene bond results in the formation of one stereogenic centre. The epoxide formed from the alkene has an optical rotation angle of -14.5° (in benzene at 589nm)[18] and has a molecular weight of 100.161 g/mol. The alkene precursor to the epoxide is available for purchase on Sigma Aldrich thus is a good candidate for further investigation.
References
- ↑ P. Caramella, P. Quadrelli, and L. Toma, Journal of the American Chemical Society, 2002, 124, 1130 DOI:10.1021/ja016622h
- ↑ M. Fox, R. Cardona, and N. Kiwiet, The Journal of Organic Chemistry, 1987, 1469–1474. DOI:10.1021/jo00384a016
- ↑ D. Skala and J. Hanika, Petroleum and Coal, 2003, 45, 105–108
- ↑ G. Bringmann, A. J. Price Mortimer, P. a Keller, M. J. Gresser, J. Garner, and M. Breuning, Angewandte Chemie (International ed. in English), 2005, 44, 5384–427 DOI:10.1002/anie.200462661
- ↑ K. Nicolaou, Z. Yang, J. Liu, and H. Ueno, Nature, 1994, 367, 630 - 634. DOI:10.1038/367630a0
- ↑ S. Elmore and L. Paquette, Tetrahedron letters, 1991, 6, 319–322. DOI:10.1016/S0040-4039(00)92617-0
- ↑ 7.0 7.1 7.2 L. Paquette and N. Pegg, Journal of the American Chemical Society, 1990, 277–283.
- ↑ W. Maier and P. Schleyer, Journal of the American Chemical Society, 1981, 1891–1900. DOI:10.1021/ja00157a043
- ↑ A. Burke and P. Dillon, Journal of the American Chemical Society, 2000, 77, 271–272. DOI:10.1021/ed077p271
- ↑ A. Vila and R. Mosquera, Journal of computational chemistry, 2007, 28, 1516–1530.DOI:10.1002/jcc.20585
- ↑ W. Zhang and J. Loebach, Journal of the American Chemical Society, 1990, 2801–2803. DOI:10.1021/ja00163a052
- ↑ 12.0 12.1 12.2 12.3 E. Jacobsen and W. Zhang, Journal of the American Chemical Society, 1991, 113, 7063–7064.
- ↑ Y. Kurosaki, T. Fukuda, and M. Iwao, Tetrahedron, 2005, 61, 3289–3303. DOI:10.1016/j.tet.2005.01.103
- ↑ 14.0 14.1 14.2 C. Wiles, M. J. Hammond, and P. Watts, Beilstein journal of organic chemistry, 2009, 5, 27. DOI:10.3762/bjoc.5.27
- ↑ D. J. Fox, D. S. Pedersen, A. B. Petersen, and S. Warren, Organic & biomolecular chemistry, 2006, 4, 3117–9. DOI:10.1039/B606881B
- ↑ H. Lin, J. Qiao, Y. Liu, and Z.-L. Wu, Journal of Molecular Catalysis B: Enzymatic, 2010, 67, 236–241.DOI:10.1016/j.molcatb.2010.08.012
- ↑ 17.0 17.1 Y. Shi, Accounts of chemical research, 2004, 37, 488–96. DOI:10.1021/ar030063x
- ↑ Ready, J. M., & Jacobsen, E. N. (2001). Highly active oligomeric (salen)co catalysts for asymmetric epoxide ring-opening reactions. Journal of the American Chemical Society, 123(11), 2687–8.
