Rep:Mod:thugaim
Module 2
Part 1
BH3
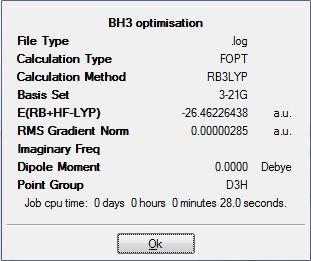
A molecule of BH3 was constructed in Gaussview 3.0 and optimized using DFT B3LYP with the following results:
B-H bond length = 1.19435Å
H-B-H bond angle = 120o
TlBr3
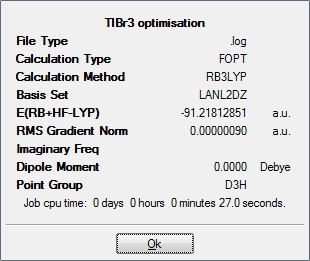
TlBr3 was confined to point group D3h and then optimised using pseudo potential LanL2DZ.
http://hdl.handle.net/10042/to-5432
Tl-Br bond distance = 2.65095Å
Br-Tl-Br bond angle = 120o
Summary:
The lit. value for Tl-Br bond distance in TlBr3 is 2.521Å[1] this value does not fall within the error of 0.01Å of the programme used for the calculation. This maybe caused by the need to use pseudo-potentials for such calculation as Tl has 81 electrons and Br has 35, needless to say, the method which we used (LanL2DZ) only has a 'medium' level of accuracy as better basis sets and pseudo potentials would require much longer calculations.
In some structures gaussview does not draw in the bonds where we expect, however this only means that gaussview does not recognise the distance between the bonding atoms when compared to its database of bond distances. A bond is simply the attraction caused by the electromagnetic force between opposing charges that allows the formation of chemical compounds.
BH3
Molecular orbitals of BH3 were calculated from the optimised structure. Digital Repository link http://hdl.handle.net/10042/to-5356
The NBO charges for boron was -0.053 and 0.018 for hydrogen.
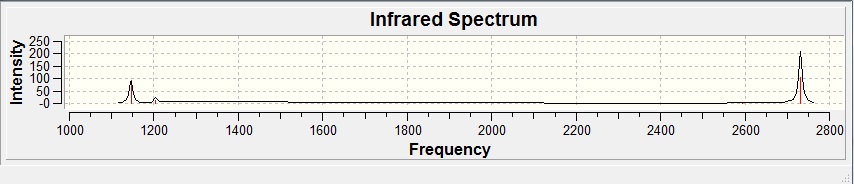
| Form Of Vibration | Freqency | Intensity | Symmetry D3h Point Group |
|---|---|---|---|
| Wagging of all 3 H atoms | 1145.71 | 92.70 | A"2 |
| Scissor or H atoms 2 and 3 | 1204.66 | 12.38 | E' |
| Rocking of all 3 H atoms | 1204.66 | 12.38 | E' |
| Symmetric stretch of all 3 H atoms | 2592.79 | 0 | A'1 |
| Asymmetric stretch of H atoms 2 and 3 | 2731.31 | 103.84 | E' |
| Symmetric stretch of H atoms 2 and 3,
asymmetric stretch of H atom 1 |
2731.31 | 103.83 | E' |
There are not 6 peaks on the spectrum because the symmetric stretch of the 3 H atoms has an intensity of 0, this is because the symmetric stretch vibration does not change the overall dipole moment.
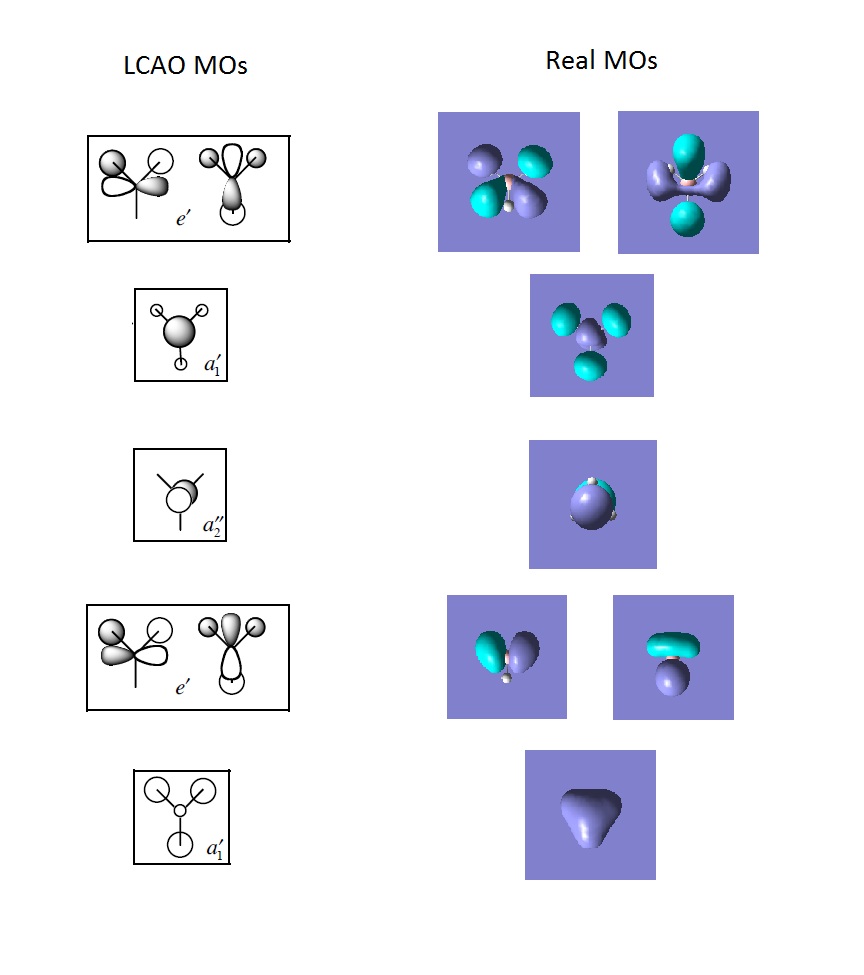
From the comparison of LCAO and Real MOs, we can see the major difference between the 2 is that the LCAO approach has a heavier focus on atomic orbitals (hence the name) while the real MOs shows electron density in a molecular fashion, having said that the qualitative approach of LCAO has accurately predicted key features such as nodal planes in all real MOs. This proves the vital importance of qualitative MO theory as quick and accurate assumptions can be made without relying on computer software.
Cis and Trans Isomers
Both trans and cis-[MoCo4(PCl3)2)] were created by fragments using gaussview 3.0. Following the procedures outlined in the script, both molecules first went under optimisation by B3LYP with pseudo-potential LanL2MB (loose convergence). Note: due to unknown complications with Dspace publishing, I have resorted to submitting job ids for publication manually, however if publication links are still unavailable by the time of marking, the job id's will be used instead as a substitute.
Cis isomer after initial optimisation: 30032
Trans isomer after initial optimisation: 30033
To avoid optimisting to the wrong minima of energy, the molecules were modified thus the cis isomer has Cl from one PCl3 group pointing up parrallel to the axial bond and Cl from the other PCl3 group pointing down; while the trans conformer had both PCl3 groups eclipsed and that one Cl of each group was parallel to one Mo-C bond. Both conformers were subjected to optimisation using a much better basis set and pseudo-potential LanL2DZ, with increased electronic convergence.
Cis isomer after final optimisation: 30034
Trans isomer after initial optimisation: 30035
In the optimised structures, the following Mo-C bond distances were obtained:
| Type | Bond Distance in Å (Cis) | Bond Distance in Å (Trans) |
|---|---|---|
| Axial | 2.05782 | - |
| Equatorial | 2.01156 | 2.06044 |
Frequency analysis was only calculated using the optimised structures of the cis and trans conformers. However due to complications, calculations did not yield valid results, therefore a separate combined opt+freq calculation was used on the initially optimised conformers, whilst retaining the better basis set and pseudo potential and increased electronic convergence keywords. This is done so that the opt+freq analysis would be identical to the result of the freq-only analysis had the calculation gone ahead successfully.
Cis isomer after opt+freq: 30135
Trans isomer after opt+freq: 30136
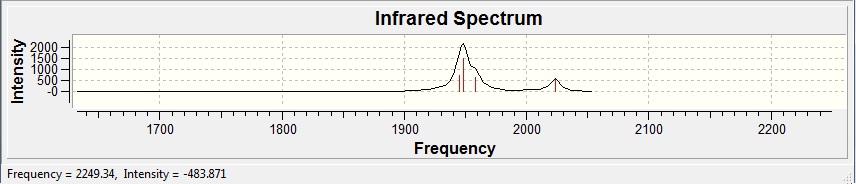
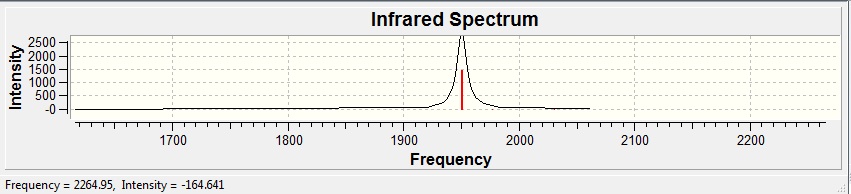
| Type | Freqency | Intensity |
|---|---|---|
| Equatorial Asymmetric | 1945.34 | 764.095 |
| Axial Asymmetric | 1948.61 | 1487.15 |
| Equatorial-Axial Asymmetric | 1958.37 | 632.266 |
| Equatorial-Axial Symmetric | 2023.27 | 598.17 |
| Type | Frequency | Intensity |
|---|---|---|
| 1,3 Equatorial Asymmetric | 1950.11 | 1474.94 |
| 2,4 Equatorial Asymmetric | 1950.71 | 1466.8 |
| 1,2,3,4 Equatorial Asymmetric | 1977.02 | 0.7215 |
| 1,2,3,4 Equatorial Symmetric | 2030.8 | 3.9078 |
Part 2 Miniproject:
Introduction
This miniproject will compare the difference in structural and electronic properties between ammonia borane and ethane. My interest in this field arose from my assigned research project 'Boron-nitrogen dehydrocoupling: a route to hydrogen storage materials'. A quick search on boron based hydrogen storage yielded a simple molecule, BH3NH3[2] to be an efficient storage medium as it can bind significant amounts of hydrogen and release under mind conditions. Ammonia borane specifically releases 13% of hydrogen in 2 steps at 100oC according to the following scheme.[3] The interesting thing to note here is that, even though ammonia borane is isoelectric to ethene, and adopts a similar structure, their boiling points differ by 284oC! Perhaps by investigating their molecular orbitals and electron density, we can somehow rationalise this fact.
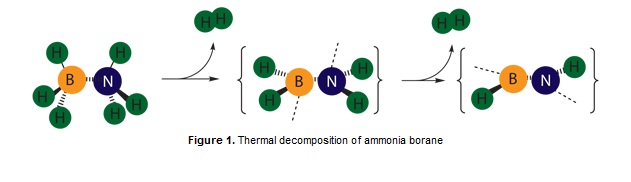
Structure
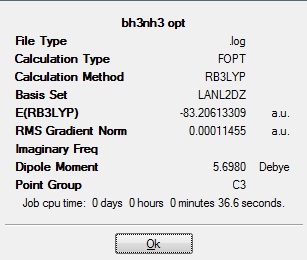
Structures of ammonia borane and ethane were minimised using B3YLP and 6-31G d,p on gaussian.
Ammonia Borane: 30486
| Bond | Length (Å) | Lit. (Å)[4] |
|---|---|---|
| N-H | 1.02213 | 1.011 |
| B-H | 1.21288 | 1.208 |
| N-B | 1.68447 | - |
| Bond | Angle (o) | Lit.(o) |
|---|---|---|
| H1-B-H2 | 113.11286 | 114.4 |
| H4-N-H5 | 110.20458 | 105.8 |
Ethane: 30487
| Bond | Length (Å) | Lit. (Å) |
|---|---|---|
| C-H | 1.07000 | 1.12 |
| C-C | 1.54000 | 1.54 |
| Bond | Angle (o) | Lit.(o) |
|---|---|---|
| H1-C-H2 | 109.47118 | 109 |
Molecular Orbitals
Below a comparison is made between the first 9 occupied orbitals and the LUMO of Ammonia Borane and Ethane.


The greatest difference between these MOs is the electron distribution around the molecules. Ethane's electron distribution is completely symmetrical around the C-C single bond, resulting in a dipole moment of 0. However, in ammonia borane, the molecule is highly polarised, this can be proved by NBO analysis which shows a much more negative charge on the nitrogen atom. The electron density also varies with most of the low lying orbitals present around the nitrogen atom. This is due to the lone pair on the nitrogen donating to the LUMO of the BH3(shown in part 1) thus changing borons hybridisation from sp2 to sp3, forming a dative bond between the N and B atoms. The evidence of the dative bond can be seen from the much longer bond length of N-B when compared to that of the length of C-C.
The donation of nitrogen's lone pair and the uneven distribution in electron density causes ammonia borane to be highly polar, more specifically causes the hydrogen atoms on the nitrogen to be more acidic. This results in 2 completely different hydrogen environments and a special type of hydrogen bonding occurs intermolecularly, also known as dihydrogen bonding.[5]
Further work was planned for this miniproject, namely the modelling and comparison of structures and molecular orbitals of BH2NH2 and BHNH, which would tell us more about why it is easy for boron to release stored hydrogen so easily, however due to running out of time no further calculations were attempted.
References
- ↑ J. Glaser et al. On the structures of hydrated Thallium(III)ion and its bromide complexes in aqueous solution 1982
- ↑ Karkamkar, A.; Aardahl, C.; Autrey, T. Material Matters, 2007, 2.2, 62
- ↑ http://www.sigmaaldrich.com/materials-science/alternative-energy-materials/boron-hydrogen-storage.html
- ↑ J. Yang et al. APPLIED PHYSICS LETTERS 92, 091916 2008
- ↑ Study of the N−H···H−B Dihydrogen Bond Including the Crystal Structure of BH3NH3 by Neutron Diffraction, DOI:10.1021/ja9825332