Rep:Mod:thegame
Modelling Using Molecular Mechanics
The Hydrogenation Of Cyclopentadiene Dimer
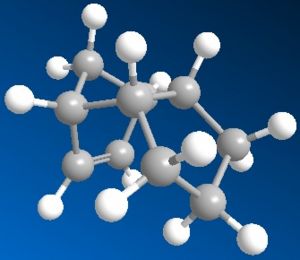
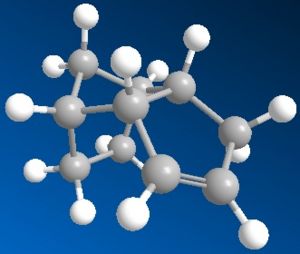
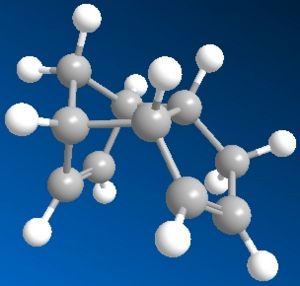
Using the MM2 method outlined in the script, both the cyclopentadiene dimers were drawn and had their total energies minimised by MM2 in Chembio3D.
| Exo-dimer | Endo-dimer | |
|---|---|---|
| Images |  |
 |
| Jmol | ||
| Total Energy | 31.8795 kcal/mol | 34.0025 kcal/mol |
However, the endo product with slightly higher energy is produced specifically through dimerisation of cyclopentadiene. This indicates that the product is not a result of thermodynamic means. Examination of the minimised molecular structure shows much less steric effect on the endo product which explains its formation over the exo product.
Through MM2 minimisations, dihydro derivative 4 produces a lower total energy. Contributions from stretching, bending, torsion, VDW as well as hydrogen bonding appears to be similar between 3 and 4, the biggest difference lies in stretch-bend, with 3 having a higher energy of 18.9414 kcal/mol compared to 4's 14.5604 kcal/mol. The difference maybe a result of better sp2 hybridization of the doubly bonded carbon in 4, when compared to a slightly compromised sp2 due to the bridging carbon on 3. It is therefore evident, that the relative ease of hydrogenation of the double bond which results in the formation of 4 is more than the other.
Stereochemistry Of Nucleophilic Additions To A Pyridinum Ring (Nad+ Analogue)
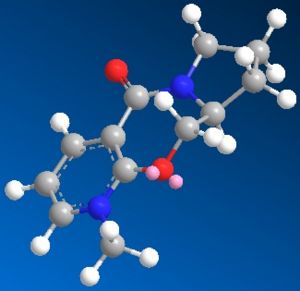
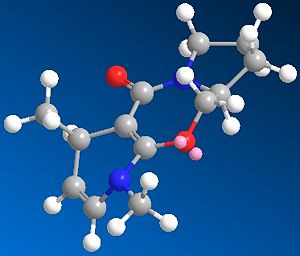
Through MM2 energy minimisation, the lowest energy conformation of 5 and 6 were found to be 43.1034 kcal/mol and 38.1476 kcal/mol respectively. Their structures are shown below:
The delivery of methyl group to 4th position of the pyridine ring is highly regio- and stereo-specific. This is caused by the strong coordination between metal centre of the gridnard reagent and the nearby oxygen atom. The conformation of reactant 5 fixes the position of the highlighted H atom perpendicular to the carbonyl oxygen, resulting in the absolute stereochemistry of product 6. The total energy is higher for the reactant 5 than product 6, however the energies of the two should not be compared as 5 and 6 are not isomers.
Similarly, the reactivity of pyridinium ring 7 can be rationalised with the same concept, which also delivers the NHPh group to the 4th position:
Pyridium 7

| Jmol | |
| Stretch | 3.9371 |
| Bend | 11.7297 |
| Stretch-Bend | 0.4078 |
| Torsion | 9.6811 |
| Non-1,4 VDW | 4.1416 |
| 1,4 VDW | 29.3203 |
| Charge/Dipole | 9.0607 |
| Dipole/Dipole | -4.8849 |
| Total Energy | 63.3933 kcal/mol |
Product 8
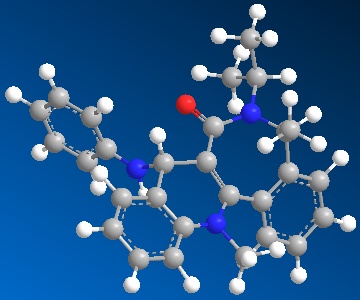
| Jmol | |
| Stretch | 4.2064 |
| Bend | 18.2939 |
| Stretch-Bend | 0.8067 |
| Torsion | 4.2748 |
| Non-1,4 VDW | 2.7393 |
| 1,4 VDW | 34.0897 |
| Dipole/Dipole | -5.9398 |
| Total Energy | 58.4711 kcal/mol |
The addition and reverse reaction which forms product 8 is highly useful in the transfer similar compounds to electrophiles through the anchor and release mechanism.[1]
Stereochemistry And Reactivity Of An Intermediate In The Synthesis Of Taxol
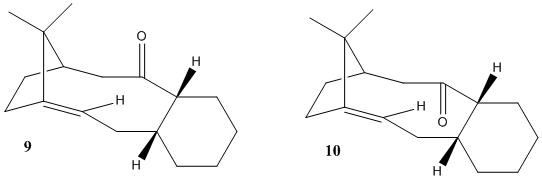
Different approaches were initiated in order to find the lowest total energy conformation for both intermediates 9 and 10, including inverting the bridge to point in the opposite direction to the O atom in intermediate 9; while inverting the bridge to point in the same direction as the O atom in intermediate 10, shown below. The highlighted H atoms in the 2D representations(right)[2] were changed from eclipsed to antiperiplanar conformation to further minimise total energy.
| Intermediate 9 | Intermediate 10 |
|---|---|
 |
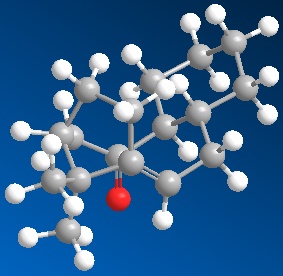 |
| Total Energy= 41.9900 kcal/mol | Total Energy= 50.3821 kcal/mol |
However, after much experimenting, the lowest energy conformations of 9 and 10 were found to be close to that of their 2D structures. These conformations have the bridging carbon pointing 'upwards', displaying significant bending to the 5 membered ring to avoid steric effects; with cyclohexane substituents assuming chair conformation. Total energies minimised by MM2 of intermediates 9 and 10 were found to be 41.7914 kcal/mol and 41.9900 kcal/mol respectively.
The contributions towards the total energies of 9 and 10 seems somewhat similar, close inspection reveals a slight difference in bending energies, which can be rationalised as the conformation of 10 is slightly more distorted to avoid steric effects between the O atom and its neighbouring ring structures. I would therefore conclude that for this specific example of atropisomerism, on standing, we would observe interconversions of intermediates 9 and 10 due to their similarities in total energy.
A similar example of atropisomerism(below) is mentioned in literature by S. W. Elmore and L. Paquette[3], where by atropselective anionic oxy-Cope rearrangement, products 5 and/or 6 could be formed from 4, with stereoselectivity being determined by the nature of X and Y. In our example of intermediates 9 and 10 no functional groups are present on the corresponding X and Y positions therefore we would not observe specific stereoslectivity towards either isomers
The term 'hyperstability' describes the characteristic in certain cyclic structures, where the strain felt by the alkene is less than its corresponding alkane.[4] In this case, the close proximity of the carbon double bond to the already strained ring structure would render it much less susceptible to subsequent fuctionalisations, resulting in abnormally slow reactivity, hence 'hyperstability'.
Modelling Using Semi-Empirical Molecular Orbital Theory
Regioselective Addition Of Dichlorocarbene
Compound 12 first undergone MM2 minimisation with the following results:
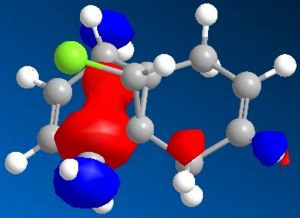
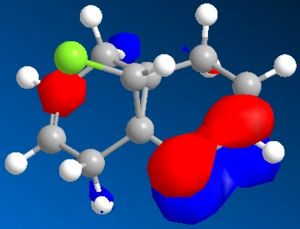
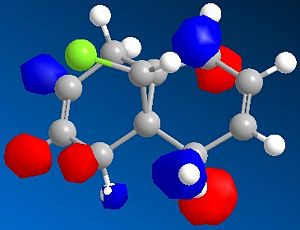
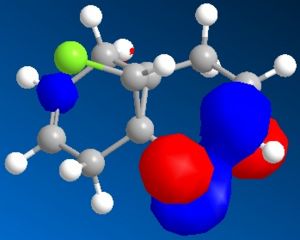
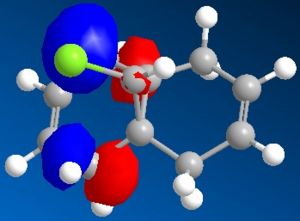
Minimisation using MOPAC/PM6 was used resulting in Heat of Formation = 19.74043 kcal/mol as well as the simulation of HOMO-2, HOMO-1, HOMO, LUMO, LUMO+1 and LUMO+2 orbitals shown below, the model of MOPAC/PM6 minimised molecule can be seen . The two can be distinguished through the angle of the endo cyclohexene plane, while it is slightly bent in the MM2 model, the angle of the MOPAC/PM6 model is planar.
| HOMO-2 | HOMO-1 | HOMO |
|---|---|---|
 |
 |
 |
| LUMO | LUMO+1 | LUMO+2 |
 |
 |
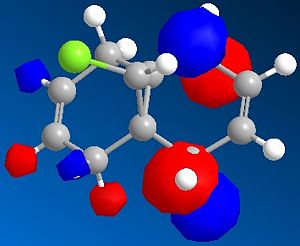 |
The simulated HOMO displays electron density on the alkene closest to the Cl atom (endo), and given that reaction with dichlorocarbene is similar to electrophilic addition, means that the endo alkene is the more nucleophilic by comparison. This observation is in agreement with literature, specifically by H. S. Rzepa et al[5], where the diene reacts regioselectively with electrophilic agents on the endo alkene, on the face of the molecule opposite the cyclopropyl ring.
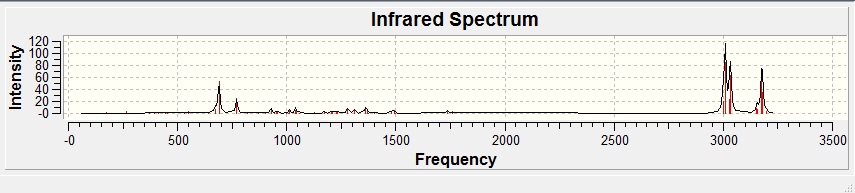
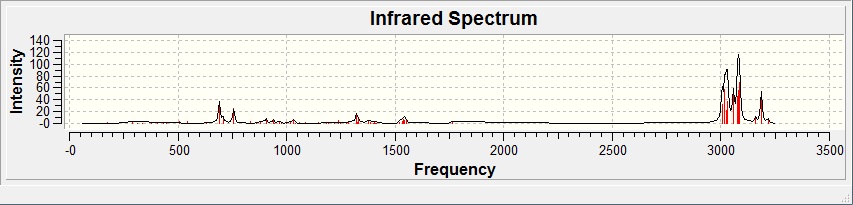
IR spectra of the Diene:
IR spectra of the Monoene:
The table below show stretching frequencies observed in both the di- and monoenes of compound 12 of C=C(endo & exo) and C-Cl bonds.
| Diene | Monoene | |
|---|---|---|
| C=C (endo) | 1757.45 | 1762.84 |
| C=C (exo) | 1737.05 | - |
| C-Cl | 770.832 | 756.818 |
Using the density functional approach, the exo and endo bonds are clearly differentiated, specifically with the endo C=C stretching frequency higher than the exo, this also implies the presence of HOMO on the endo alkene. Note that there is also a minor difference in the stretching frequencies of C-Cl bonds between the di- and monoene.
Mini Project - Stereoselective Dissolving Metal Reductions
Introduction
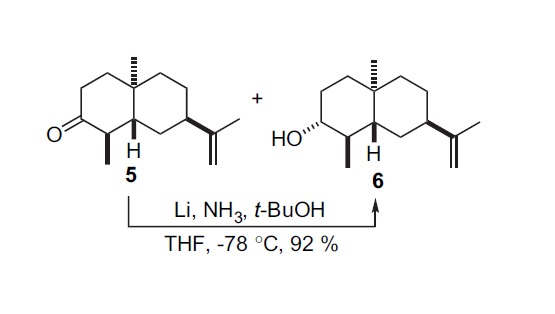
This mini-project is based around stereoselective dissolving metal reductions through Birch reduction. Specifially, the reduction of ketone 5 to alcohol 6 shown in the reaction scheme(right), this reduction reaction uses dissolving metal Li/NH3 and t-BuOH as the proton doner, at -78oc in THF, the reaction is highly stereoselective, and achieves a 92% yield.
This project uses molecular modelling techniques such as MM2 and DFT minimise molecule geometry as well as Gaussian to predict NMR, IR spectra and Optical Rotation. These methods are used to analyse both the starting material and product, confirming their existence and physical properties, which are compared with data obtained in literature[6], allowing the achievement of similar results using only computer software.
Reaction Scheme
The high stereoselectivity is a combination of factors. The coordination of the metal ion with the equatorial carbonyl oxygen on this enolizable, saturated ketone allows the formation of the more stable, cis-enol[7]/anion[8](examples of both enol formation and dianion mechanism are shown below).
Subsequent axial protonation of the carbonyl carbon by t-BuOH occurs on the plane opposite of the propene substituent. The coordinating effects of the metal ion ensures that the equatorial conformation of OH group on the final product is maintained.
Minimised Conformations
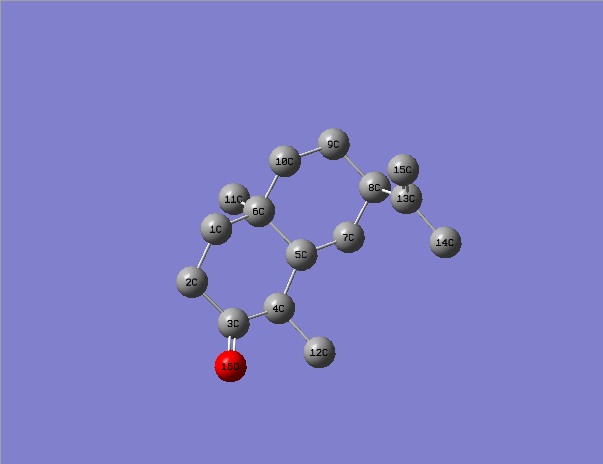
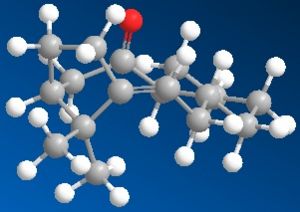
Using ChemBio3D, models of ketone 5, alcohol 6 and alternative stereoisomer alcohol 7 were constructed and minimised with mm2. Their structures and total energy are shown in the table below. As all molecules in question contain only simple elements and a total of <50 non hydrogen atoms, MM2 was sufficient in pre-Gaussian minimisations. These structures were then processed by Gaussian using method DFT=mpw1pw91 as it was tested to be superior to B3lYP procedure in the calculation of NMR properties.[9]
| Ketone 5 | Alcohol 6 | Alcohol 7 | |
|---|---|---|---|
| 2D Images | 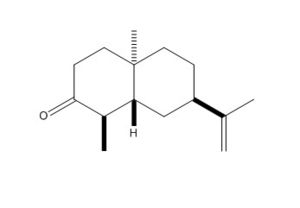 |
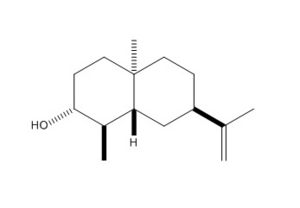 |
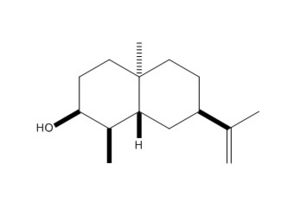 |
| 3D Images | 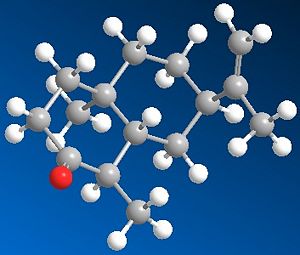 |
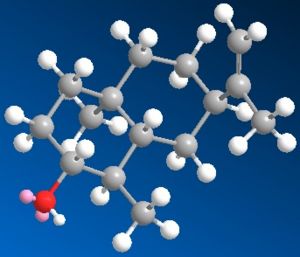 |
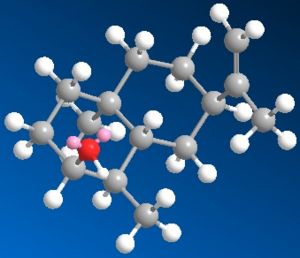 |
| MM2 Total Energy | 24.6561 kcal/mol | 24.5413 kcal/mol | 24.9069 kcal/mol |
| DFT Minimised Jmol |
13C NMR Spectra
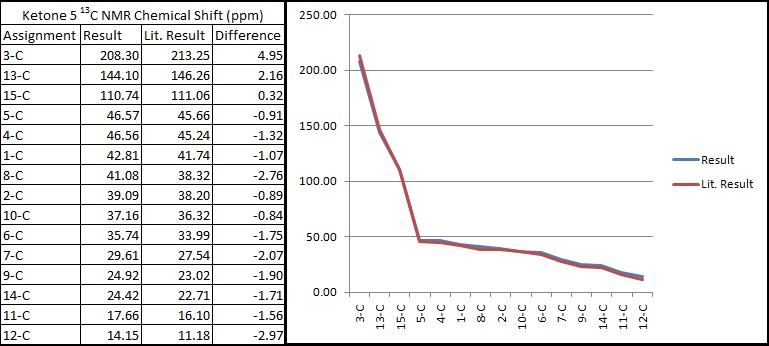
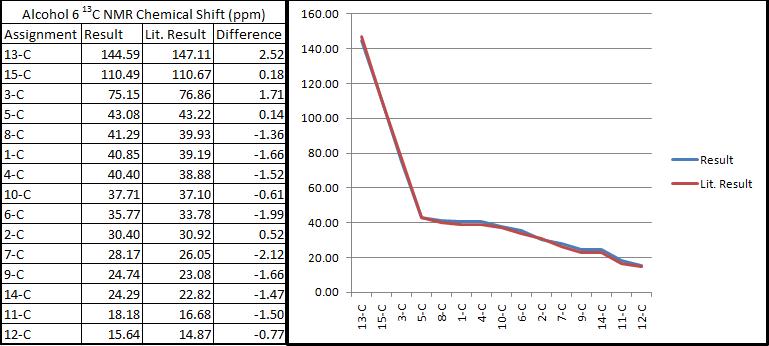
After DFT minimisation, molecules of 5 and 6 were submitted for NMR calculation. Below are the 13C spectra, tabulated data comparison and Digital Repository URL of molecules 5 and 6 respectively, the reference value used was 'TMS mPW1PW91/6-31G(d,p) GIAO'. NMR calculation was not done for molecule 7 however, as 7 and 6 share the same NMR properties.
Ketone 5
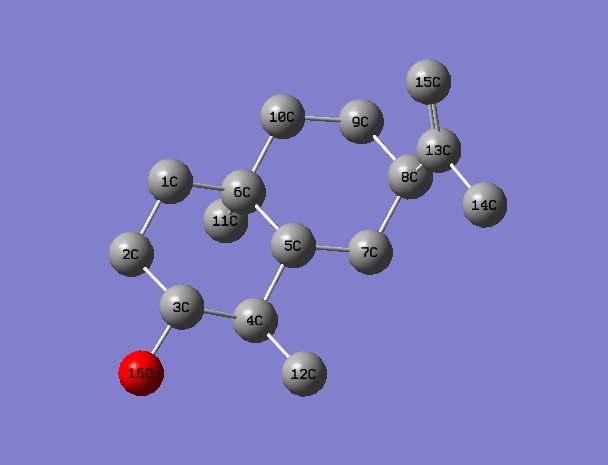
Carbon atoms on molecule 5 are assigned in the following fasion:
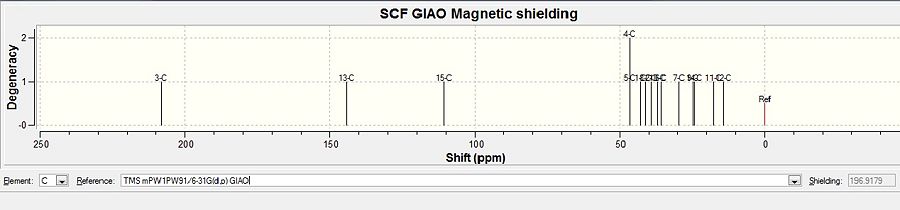
Alcohol 6
Carbon atoms on molecule 6 are assigned in the following fasion:
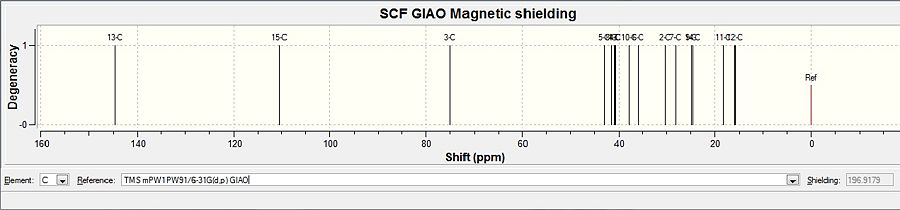
As can be seen from the comparison graphs, the calculated NMR chemical shifts are very close to literature values, this indicates that the conformation adopted by minimisations of the molecule had indeed been correct, and also confirms that the reduction process in literature had successfully taken place experimentally according to the reaction scheme. Published NMR calculation can be found for molecule 5 here, and for molecule 6 here.
IR Spectra
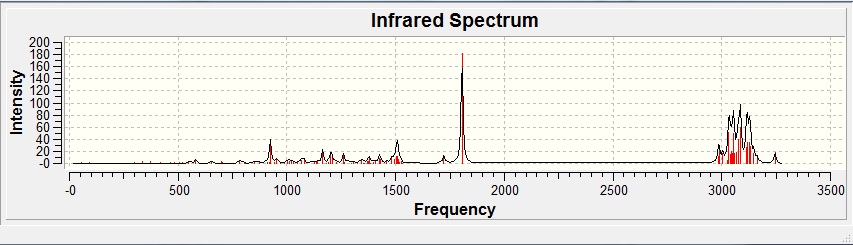
IR predictions were obtained in a single job from MM2 minimised conformations of compounds 5 and 6 using b3lyp/6-31G(d,p).
Predicted IR Spectrum Of Ketone 5:
Predicted IR Spectrum Of Alcohol 6:
| Ketone 5 | Alcohol 6 | |
|---|---|---|
| C=C | 1721.97 | 1722.26 |
| C=O | 1805.51 | |
| C-O | 1084.97 | |
| O-H | 3833.94 |
Upon inspection, it is trivial to differentiate and identify compounds 5 and 6 from their IR spectra. Compound 5 possesses a C=O stretch which gives a very sharp, high intensity peak at 1805.51cm-1, similarly compound 6's alcohol group gives off low intensity peaks at 1084.97cm-1 and 3833.94cm-1 which is not present on the ketone spectrum. No predicted IR spectra of 7 was calculated as its results would be the same as 6.
Optical Rotation
Unfortunately due to running out of time, calculations for optical rotations of 6 and 7 was not completed. However, if one was to calculate the optical rotations, one expects to find opposite values of [alpha]D for 6 and 7 as the OH group points away from the plane of the ring structure at different angles, in this case 6 has the OH group in the equatorial position while 7's OH group is in the axial position. The [alpha]D20 (c 1.02, CHCl3)value given in literature for compound 6 is -11.6, therefore the [alpha]D20 value for 7 is predicted to be at +11.6.
Conclusion
This mini project has, to some extent, produced plausible data and calculations that match up and confirms experimental findings. By making use of MM2, DFT minimisation methods, reasonable conformations of molecules were discovered and used in 13C NMR and IR predictions. However, as Optical Rotation results were not obtained, it had not been possible to confirm that this Birch reduction of ketone 5 is in fact stereoselective towards alcohol 6. This can neither be backed up thermodynamically with total energies calculated by MM2 as 6 and 7 had 24.5143 kcal/mol and 24.9069 kcal/mol respectively, an insignificant difference which does not evidently indicate stereoselectivity.
Summary
By working through the assignments in this module, I have gained an insight to various molecular modelling and spectra predicting methods. Moreover I have learnt the importance of these techniques in understanding and interpreting properties of small molecules, and the astonishing accuracy of these techniques makes their usage vital in any aspect of chemistry. Hopefully with better time management and more familiarity with wiki editing, the quality of future projects can be improved.
References
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- ↑ http://www.ch.imperial.ac.uk/wiki/index.php/Main_Page
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ W. Maier, P. Schleyer., Evaluation and prediction of the stability of bridgehead olefinsJ. Am. Chem. Soc., 1981, 103 (8), pp 1891–1900 DOI:10.1021/ja00398a003
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ L. Castellanos et al. Stereoselective synthesis of (-)-4-epiaxinyssamine. 2007 DOI:10.1016/j.tet.2006.12.019
- ↑ J. Huffman. et al. Dissovling Metal Reductions Of Ketones: Comments On The Dianion Mechanism, Tetrahedron Letters. 1987. 3315-3318 DOI:10.1016/S0040-4039(00)95500-X
- ↑ M. Robinson. Conformational effects in compounds with six-membered rings-V: Reduction of unsaturated ketones by dissolving metals. Tetrahedron 1965. 2475-2487. DOI:10.16/S0040-4020(01)93903-1
- ↑ http://www.ch.imperial.ac.uk/wiki/index.php/mod:organic