Rep:Mod:thatssoraven1b
Module 1: Mini project
Personal Mini Project - Siegfried Stow
Background
In the synthesis of the Antibiotic Neoxazolomycin A [1] the authors decided to synthesise the molecule in three parts, with the lactone with several stereochemical centres on the right being the most challenging. The authors made the disconnection on the left of the alcohol stereocenter, and protect the alcohol with a Benzyl group to give the target fragment, named in the paper and here as [3].[1]

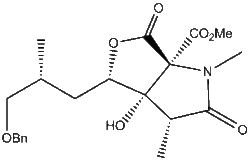
The key step to form [3] with most of its stereocenters is a Dihydroxylation of [4] (using OsO4 in THF with NMO as oxidant) to form two stereochemical centres and lead to the formation of the lactone ring[2] . Nothing in the mechanism suggests that the OSO4 will be stereoselective for either side, although the neighbouring methyl group suggests there might be a very small preference for the OSO4 to attack on the face opposite the methyl group. For clarity, the face with the methyl group will be referred to as ALPHA and the face opposite the methyl group will be BETA. Pictured: The formation of the ALPHA dihydroxylated product [3] from the alkene [4], then the formation of the BETA isomer
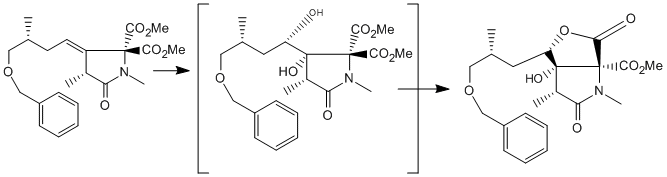
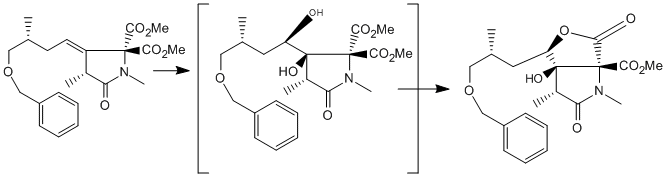
The alkene could be attacked from either of its two faces, but the reaction reportedly proceeds with quantitative yield for the desired ALPHA dihydroxylated product [3]. Once Dihydroxylated, the alcohol reacts with one of the methyl esters to form the lactone. This is attributed by the authors to the interaction of the benzyl protecting group and the less hindered BETA face of the alkene, leaving the slightly more hindered ALPHA face open to dihydroxylation. Their speculation is that in the THF solvent the benzyl group wraps around the molecule in order to form a favourable interaction with the double bond[3], and that it is able to form a better interaction with the BETA face than it can with the ALPHA face, which has a methyl group near it. this sort of 'pi-stacking' interaction is common in enzyme active sites, where such interactions can account for 2-4kjmol. What is astounding about this hypothesis is that the benzyl group not only protects the less hindered BETA face preferentially, but that it protects it quantitatively.
Furthermore, in enzyme active sites the active site is a non-polar haven, separate from the highly polar aqueous solvent in which the enzyme operates. Thus very favourable pi-stacking interactions can result[4]. in the dihydroxylation reaction to form [3] however the solvent is non polar THF, and the neighbouring methyl ester groups are marginally polar and contribute steric hindrance to the large benzyl group, so the interaction isn't worth a great deal of energy.
The Project
In order to gain insight into the problem the energies of the conformations of the benzyl group will be modelled with high level theory and compared, in order to gauge what populations of the two conformers exist and therefore how likely the reaction is to be stereospecific in the dihydroxylation.
Furthermore, the two possible dihydroxylation product lactones (ALPHA [3] & BETA) will be compared by modelling them and finding their minimum conformation energies.
The 13C NMR spectra of the two ALPHA and BETA dihydroxylation product lactones will also be simulated and their peaks compared with those reported in the literature.
Once Dihydroxylated, the alcohol reacts with one of the methyl esters to form the lactone. as there are two methy esters at the neighbouring carbon center, there are two possible lactones, 'cis' and 'trans'. typically Trans lactones have ~20kjmol higher energy than cis lactones, so the trans lactone isomers will not be considered (c.f molecules C and C', D and D' from part 4: glycosidation).
Conformations
In the Paper, the authors attribute the unusual selectivity to a pi stacking interaction between the double bond and the benzyl protecting group. Therefore it follows that the pair should interact via frontier molecular orbitals. The Benzyl chain can exist in a large number of conformations with a large number of local minima, so the calculations were started with the benzene ring in a favourable conformation, and then the structure minimised. Molecular orbitals were also calculated, in order to understand the pi stacking interaction. the MOPAC PM1 system was used to produce the MOs as it uses less CPU time and there were many configurations to test. the Conformations produced were in good agreement with the conformations produced by Gaussian.
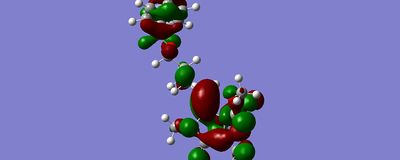
With the Bn group position directly infront of the Alpha face (clashing with the methyl group) the energy minimisation converged with the Bn well away from the alkene, at -225.876kj/mol:
converged ALPHA face |
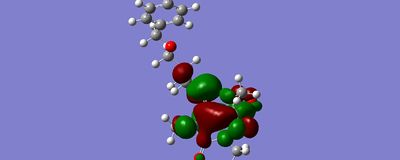
the Bn group was then re-arranged so that it would have perfect pi-stacking interactions with the more accessible BETa face. Once again, the minimisation converged well away from the double bond, this time at -230.82kcal/mol.
converged BETA face |
Whilst the lowest energy conformation found by the programs was not the expected pi stacking interaction, orbitals produced by gaussian show that there is a possible HUMO LUMO interaction between the Benzyl group and the double bond:
the calculations were done in vacuum conditions rather than a solvent situation, which is likely to have prevented the program from finding the wrapped round pi stacking conformation. further simulations would be done with the COSMO solvent approximation. The simulations indicate that the benzyl group does not interact signigicantly with either face of the alkene. whilst pi-stacking is observed between benzene rings in the gas phase at seperations of 5A, the benzyl group is too poorly aligned in the simulations. neither the face nor the edge of the ring point toward the alkene, and the benzyl group is considerably dispaced.
dihydroxylation and lactone energies
| Isomer | Energy / Hartree |
|---|---|
| ALPHA | -1397.57065961 |
| BETA | -1397.55718484 |
the energy difference between the two isomers shows that the Beta isomer (not produced) is 3.54kj/mol lower in energy than the Alpha Isomer that is produced. this suggests that if the reaction is thermodynamically controlled then the reaction should result in 99.9% Beta product. evidently the reaction proceeds kinetically. OsO4 does not react using equilibrating conditions, so the reaction is not expected to proceed thermodynamically.
13C NMR spectra
The 13C NMR spectra generated from optimised structures using "mpw1pw91/6-31g(d,p)" optimisation, a DFT method. the GIAO method is then used to generate the NMR shifts, and compared to a simulated tms reference.

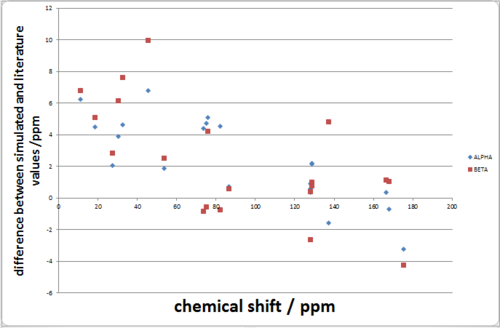
| Literature (alpha) | Simulated Alpha | Simulated Beta |
|---|---|---|
|
175.2 167.7 166.2 137.2 128.5 128.5 128 127.9 127.9 86.5 82 75.8 75.1 73.4 53.5 45.4 32.6 30.3 27.3 18.4 11 |
171.9742 167.0192 166.5892 135.6525 130.6878 130.6743 128.8965 128.5026 128.285 87.2254 86.5722 80.8871 79.8457 77.8123 55.3728 52.1927 37.2409 34.218 29.3832 22.8832 17.2492 |
170.9511 168.7499 167.3481 142.0338 129.4948 129.2713 128.433 128.3236 125.2918 87.086 81.2747 80.042 74.5776 72.5728 56.0425 55.3894 40.2387 36.4593 30.1688 23.5067 17.8 |
| average error | 3.08 | 3.32 |
The difference in the NMR simulations and the literature values are too similar to make any meaningful conclusions whether the correct isomer was isolated in the original paper.
conclusion
The computations undertaken were insufficent to confirm or reject the literature hypothesis that the neighbouring benzyl group participates in the reaction to confer selectivity. The Benzyl group can have orbitals of the correct energy and alignment to interact beneficially with the double bond's pi orbitals, but simulation shows that the most stable conformations are too far away from the alkene or too poorly aligned to actually interact.
the product energies suggest that the beta isomer is more stable then the observed alpha isomer, which suggests that the reaction must proceed via a kinetic pathway, so that the selectivity must be entirely down to more facile access to the alpha face for the OSO4 reactant species.
The NMR predictions were too similar to afford a confirmation of either structure based on the chemical shifts.
Overall, the work in this project weakly rejects the original hypothesis in the literature that there is neighbouring group participation by the benzyl group. Calculations of the level undertaken in this project suggest that the lowest energy conformation does not permit participation by the benzyl group. Instead it is more likely that there is differential solvation at the two sites by THF, or that there are more important steric interactions from the methyl ester groups that allows the reaction to distinguish between the two faces.
To further improve this work calculations which include solvent effects would be undertaken.
References + additional information
The Fchck .out .mol files for some of the calculations
- [4] - the double bonded structure, started with benzyl on alpha face
- [4] - the double bonded structure, started with benzyl on beta face
- [3] - dihydroxylated on alpha face
- [3] - dihydroxylated on beta face
References
- ↑ Total Synthesis of Neooxazolomycin, Evans Otieno Onyango, DOI: 10.1002/anie.200702229
- ↑ Recent developments in metal-catalyzed dihydroxylation of alkenes By: Prof. Dr. Jan-Erling Bäckvall, DOI: 10.1002/9783527632039.ch1
- ↑ J. Am. Chem. Soc., 2002, 124 (36), pp 10887–10893
- ↑ The organic chemistry of enzyme-catalyzed reactions Silverman, Richard B.