Rep:Mod:testhlj298
Cyclohexa-1,3-diene and Maleic Anhydride
First, the structures of the reactants, ethylene and cis-butadiene, were optimised to minimum under semiempirical AM1 method. Details are listed in Table 17.
Table 17. Summary of the optimized reactant
Then, the two transition states, exo and endo, of the reaction were able to construct. By making the bond lengths of the bonds to be made in Diels-Alder [4+2] cycloaddition to 2.20000 Å in the transition state, which is the 'dash bond', the transition structure was optimized to Opt + Freq minimisation to TS (Berny) under the same method of calculation - semiempitical AM1. Details are listed in Table 18.
Table 18. Summary of the optimized transition structures
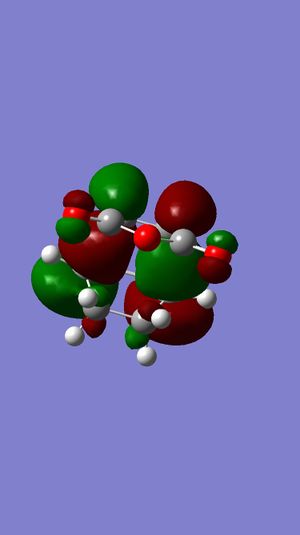
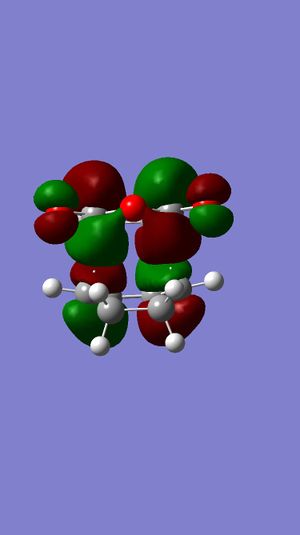
In Table 18, we can see that both HOMO and LUMO of the exo transition state are antisymmetric with respect to the plane. Also, both HOMO and LUMO of the endo transition state are antisymmetric as well.
Also, we notice that the energy of exo is higher than that of endo. This can be explained by the poorer overlap between the C=C π and C=O π* compared to that of endo. This is called secondary orbital effect, which will be further discussed below.
There is one imaginary frequency in both transition structure. This confirms that both of them are valid postulated transition structures.
| JSMOL
Imaginary frequency of exo transition state animation |
JSMOL
Imaginary frequency of exo transition state animation |
Again, from the above animations, we can see that the bonds are formed in a synchronous fashion, which means the Diels-Alder Cycloaddition reaction is concerted.
Furthermore, the geometries of both transition states were examined carefully in Table 19.
| Geometry summary of Exo Transition State (Please refer to Figure 43 for atom labelling) | Geometry summary of Endo Transition State (Please refer to Figure 44 for atom labelling) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 19. Geometry analysis of exo and endo transition states
According to the reaction scheme shown in Figure 4, a single bond is forming between C5 and C13, also another single bond is forming between C6-C14 for exo; C15 and C7 plus C16 and C5 for endo, which is what the first row in the two tables in the left and right in Table 19 shows. the single bond to be made Comparing these values with literature, we find that they are longer than C-C but shorter than twice of carbon's Van der Waals' radius. This tells us some hints that these pairs of carbons between two reactants are attracting with each other to a certain degree. Furthermore, taking a look to other carbon-carbon bonds within the system, i.e. except row 1 and those labelled with (through space), we notice that they are somewhere between C-C and C=C. This tells us that either C-C is becoming C=C or C=C is becoming C-C in the transition state.
Now, looking at the through space bond length. In the exo transition structure, the through space distance -(C=O)-O-(C=O)- fragment of the maleic anhydride and the C atoms of the “opposite” -CH2-CH2- is smaller than the twice of the Van der Waals radius but larger than C-C which gives us a hint that they might interact. In the endo transition structure, the through space distance -(C=O)-O-(C=O)- fragment of the maleic anhydride and the C atoms of the “opposite” -CH=CH- is smaller than the twice of the Van der Waals radius but larger than C-C which gives us a hint that they might interact. However, according to the definition of secondary orbital effect, it is looking for the interaction between the C=C π of the diene and C=O π* of the dienophile. Endo clearly shows that as explained, but exo seems to just demonstrate the sterics clash between the -(C=O)-O-(C=O)- fragment of the maleic anhydride and the C atoms of the “opposite” -CH2-CH2- fragment of diene. In order to further confirm that exo has no secondary orbital effect, a measurement of bond length was carried out between -(C=O)-O-(C=O)- fragment of the maleic anhydride and the -CH=CH- in diene in the exo transition state. The result was shown in the last row on the left table in Table 19. This shows that they are too far away which means they are not possible to interact.
Now, looking back to the HOMO of exo and endo transition states in Figure 45 and 46 respectively. We can definitely see that the overlap between the two reactants is relatively smaller in exo. From these two pieces of information, we can conclude that the endo is kinetically controlled, while exo is thermodynamically controlled.
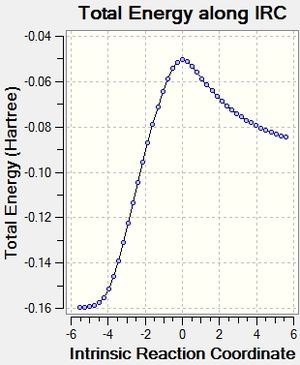
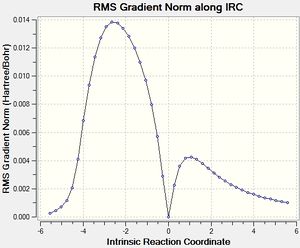
After that, the IRC of the both optimised transition state was carried out with both direction and force constant calculated always for the reaction profile. 21 points were used for exo transition states and 24 for endo (reasons explained under Introduction) to see the reaction profiles.
And eventually, the activation energies of the reaction via different transition structures were summarised in Table 20.
| Cyclohexa-1,3-diene | Maleic Anhydride | Endo Transition State | ExoTransition State | Activation Energy via endo | Activation Energy via exo | |
|---|---|---|---|---|---|---|
| Energy/Hartree | 0.02771130 | -0.12182418 | -0.05150469 | -0.05041984 | 0.04260819
(26.74 kcal/mol) |
0.04369304
(27.42 kcal/mol) |
Table 20. Activation energy analysis