Rep:Mod:test page AC
NH3 molecule
N-H bond distance= 1.01798 Angstroms
H-N-H bond angle= 105.741 degrees
Calculation method= RB3LYP
Basis set= 6-31G(d.p)
Final energy= -56.55776873 au
RMS gradient= 0.00000485 au
point group= C3V
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 molecule |
File:AC NH3 OPTF POP.LOG The optimisation file is liked to here
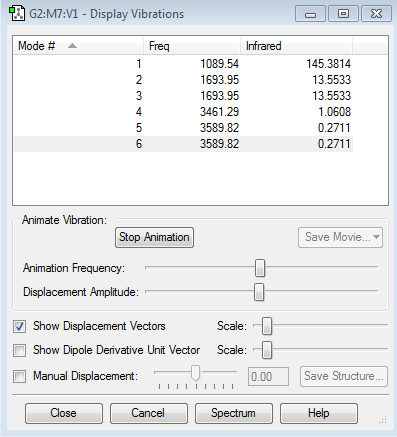
Questions how many modes do you expect from the 3N-6 rule? A:N=4 therefore modes=6
which modes are degenerate (ie have the same energy)? A: modes 6 and 5 and modes 2 and 3 have the same energy(degenerate) as they have the same frequency.
which modes are "bending" vibrations and which are "bond stretch" vibrations? A: mode1: bond bend, mode2: bond stretch, mode3: bond bend, mode4: bond stretch, mode5: bond stretch, mode6:bond stretch
which mode is highly symmetric? A: mode1
one mode is known as the "umbrella" mode, which one is this? A: mode1
how many bands would you expect to see in an experimental spectrum of gaseous ammonia? 5 bands because the 4th mode is a symmetric stretch.
charge on N atom: -1.125, charge on H atom:0.375. The N would be expected to have a negative charge and the hydrogen positive due to Nitrogen being the more electronegative of the two atoms, pulling electrons in the covalent bond towards itself hence having a negative charge.
N2 molecule
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Final energy: -109.52412868 au
RMS gradient: 0.00000365 au
Point group: D∞H
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES
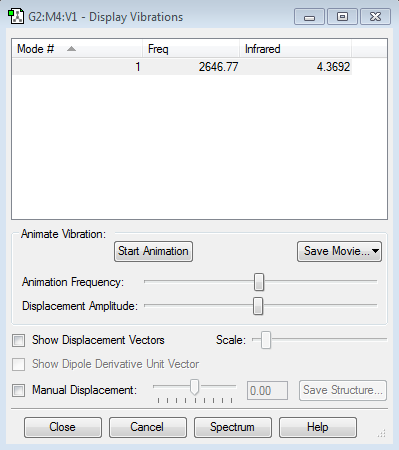
N2 molecule |
Frequency for N2 molcecule: 2457.31, this confirming there is no negative frequencies. And satisfying that there should be one frequency. (3x2)-5=1
H2 molecule
Calculation method: RB3LYP
Basis Set: 6-31G(d.p)
Final energy: -1.17853936 au
RMS gradient: 0.09719500 au
Point group: D∞H
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 molecule |
Determining the energy of the reaction of N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 au
2*E(NH3)= -113.1155375 au
E(N2)= -109.52412868 au
E(H2)= -1.17853936 au
3*E(H2)= -3.53561808
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0557904 au -146.47770636 kJ/mol
It can be seen that the product of ammonia is more stable than the gaseous reactants as there is a release of energy (exothermic reaction) showing that product is lower in energy and therefore more stable.
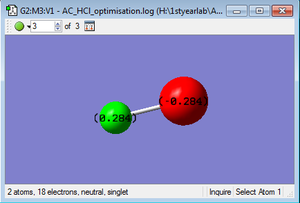
Project molecule- HCl
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Final energy: -460.80077875 au
RMS gradient: 0.00005211 au
Point group: C∞V
Item Value Threshold Converged? Maximum Force 0.000090 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000139 0.001800 YES RMS Displacement 0.000197 0.001200 YES
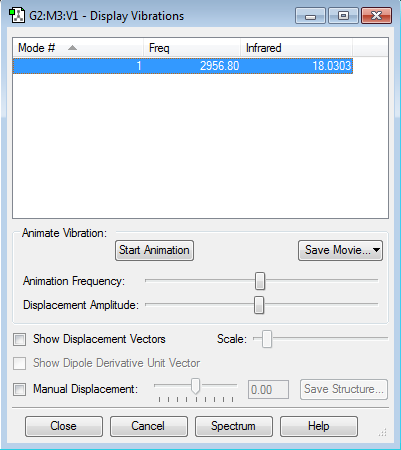
HCl molecule |
As seen by the image above there is only one mode. This is consistent with the expected value (3x2 -5=1) for a linear molecule with two atoms.
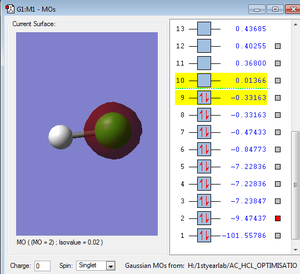
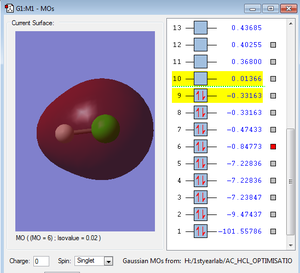
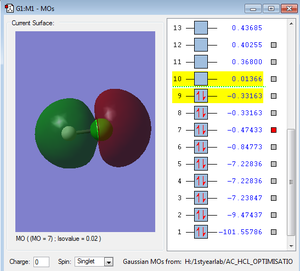
Orbitals

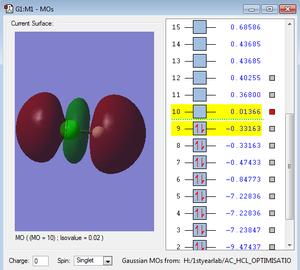
HBr molecule
Calculation method: RB3LYP
Basis Set: 6-31G(d.p)
Final energy:-2572.30644608 au
RMS gradient: 0.00000078 au
Point group: C∞V
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000003 0.001800 YES RMS Displacement 0.000004 0.001200 YES
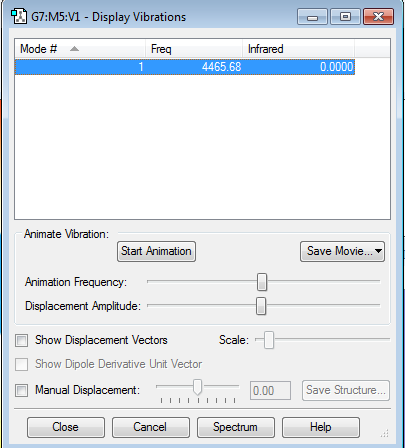
HBr molecule |
Comparison to HCl
The optimized energy for HCl is -460.80077875 au and for HBr it is -2572.30644608 au, this could be due to the reduced reactivity of HBr relative to HCl. This could be explained by the larger dipole moment that HCl has relative to HBr.