Rep:Mod:test1111
Appearance
Frequency Analysis
Using the 3N-6 rule, you would expect 6 vibrational modes. Modes 2 & 3 are degenerate and modes 5 & 6 are also degenerate. Modes 1, 2 & 3 are bending. Modes 4, 5 & 6 are stretching. Modes 4 is highly symmetric. Modes 1 is known as the 'umbrella' mode. In an experimental IR spectrum of gaseous ammonia 2 peaks would be present. This can be seen from the intensities of the vibrations, the first mode is very intense, modes 2 & 3 are degenerate, with a reasonable intensity.
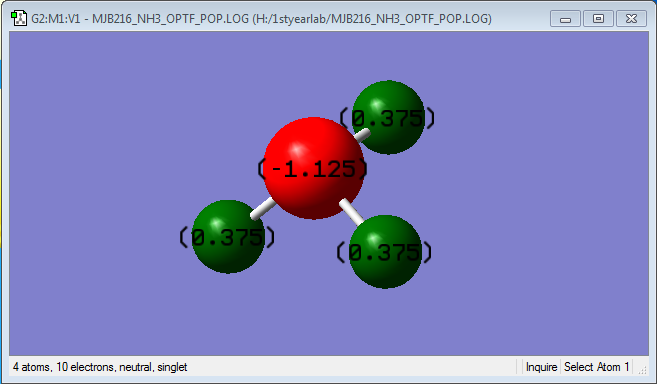
Charge Distribution
As Nitrogen is a more electronegative atom than Hydrogen, it would be expect that the nitrogen atom would withdraw some electron density from the N-H bonds towards it, hence giving it a negative charge.