Rep:Mod:teetyso
EX3
BH3
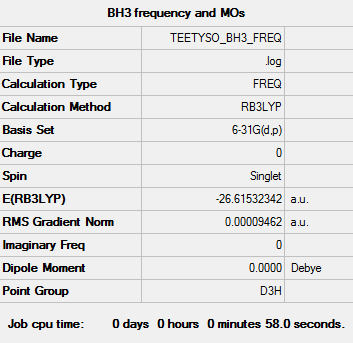
Key data for BH3
B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000189 0.000450 YES RMS Force 0.000095 0.000300 YES Maximum Displacement 0.000746 0.001800 YES RMS Displacement 0.000373 0.001200 YES
Low frequencies --- -0.2263 -0.1037 -0.0055 47.9770 49.0378 49.0383 Low frequencies --- 1163.7209 1213.6704 1213.6731
Click here to link to File:TEETYSO BH3 FREQ.LOG
BH3 Molecule |
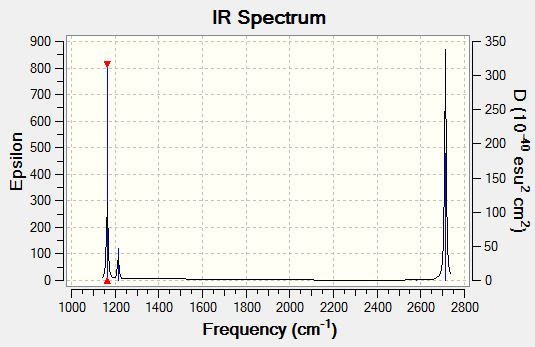
Infrared Information for BH3
| Mode # | Wavenumber (cm-1) | Intensity (arbitrary units) | Symmetry | IR Active? | Type of Vibration |
|---|---|---|---|---|---|
| 1 | 1163 | 92 | A2" | Yes | Out of plane bend |
| 2 | 1213 | 14 | E' | Yes | Bend |
| 3 | 1213 | 14 | E' | Yes | Bend |
| 4 | 2579 | 0 | A1' | No | Symmetric stretch |
| 5 | 2712 | 126 | E' | Yes | Asymmetric stretch |
| 6 | 2712 | 126 | E' | Yes | Asymmetric stretch |
From computational calculations, it is found that there are six vibrations. However, there are less than six peaks in the spectrum. This is due to the fact mode number 4, is an in-plane stretch where all hydrogen atoms are moving in and out in synchronization with each other. Consequently, there is no change in dipole moment and hence will not give rise to a signal in the IR spectrum. In addition, mode 2 and 3 have the same frequency (hence same energy), as does mode 5 and 6. These are two sets of degenerate vibrations and will only give rise to one signal each.
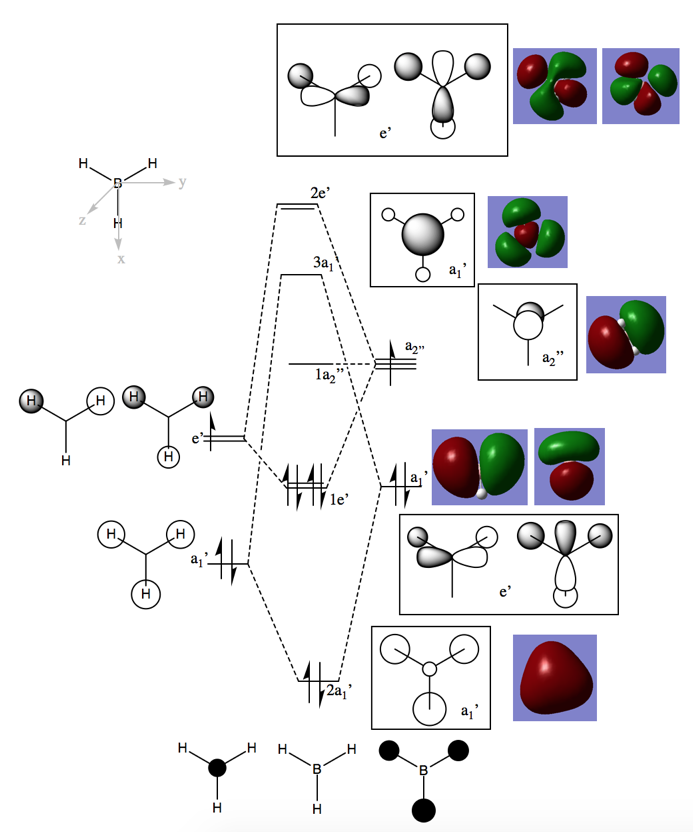
Visualizing BH3 Molecular Orbitals
Comparing the real and LCAO MOs, the computed orbitals give good comparisons in terms of the overall shape, size and phases present. This demonstrates that LCAO gives a good depiction of what the BH3 molecular orbitals look like, and is useful in visualizing the interactions of orbitals. However, it is important to note that the accuracy of this system may be high due to the fact that this system is comprised of light atoms and a low number of electrons, thus relativistic effects may not be significant. Moreover, it is seen that the computed molecular orbitals do not compare well with the LCAO when looking at unfilled orbitals.
Ng611 (talk) 18:18, 21 May 2018 (BST) Clear inclusion of the MOs on the diagram and both similarities and differences between the LCAOs and MOs considered. Well done.
NH3
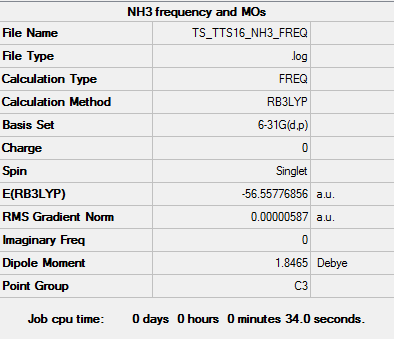
Key data for NH3
B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
Low frequencies --- -8.5646 -8.5588 -0.0047 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
NH3 Molecule |
Have you added the key data for NH3? Have you added the key data for NH3BH3? Have you included your energy calculations? Have you answered the bond strength questions?
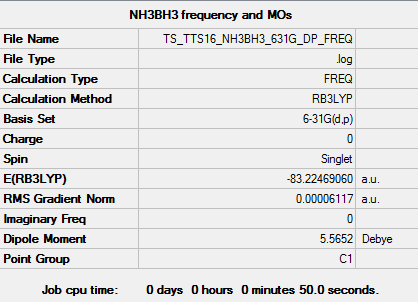
NH3BH3
Key data for NH3BH3
B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000113 0.000450 YES RMS Force 0.000061 0.000300 YES Maximum Displacement 0.000659 0.001800 YES RMS Displacement 0.000441 0.001200 YES
Low frequencies --- -0.0007 -0.0003 0.0013 17.6705 28.0332 40.2175 Low frequencies --- 266.5190 632.3638 639.4914
File:TS TTS16 NH3BH3 631G DP FREQ.LOG
NH3BH3 Molecule |
Determining the bond association energy of the B-N bond
Calculation of the association energy of the B-N Bond:
E(NH3): -56.55777 au
E(BH3): -26.61532 au
E(NH3BH3): -83.22469 au
ΔE = E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - (-56.55777 - 26.61532) = -0.05160 au (5 d.p) = -135 kJ/mol
Based on the above energy calculations, the B-N dative bond is a relatively weak bond, as to a C-C single bond has an energy dissociation of -348 kj/mol. Furthermore, boron and nitrogen have an electronegativity difference of 1.0, thus giving rise to a polarized system which may allow the bond to break more readily, compared to that of a carbon-carbon bond
Ng611 (talk) 18:19, 21 May 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper).
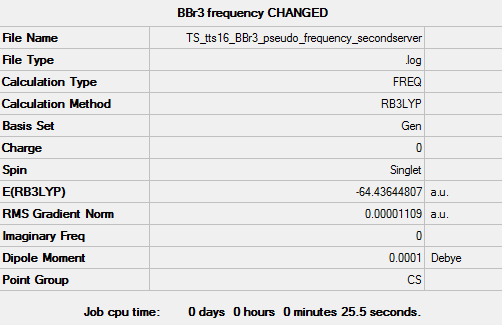
BBr3
Item Value Threshold Converged? Maximum Force 0.000025 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000109 0.001800 YES RMS Displacement 0.000048 0.001200 YES
Low frequencies --- -4.3208 -2.7694 -2.3007 0.0001 0.0001 0.0002 Low frequencies --- 155.8708 155.9431 267.6977
File:TS tts16 BBr3 pseudo frequency secondserver.log
BBr3 Molecule |
Project Work
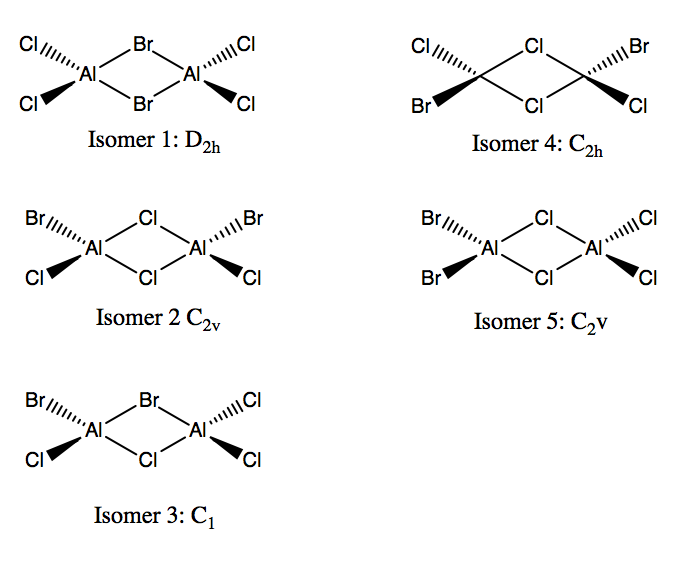
For this part of the project, Al2Br2Cl4 was investigated.
Possible Isomers of Al2Br2Cl4
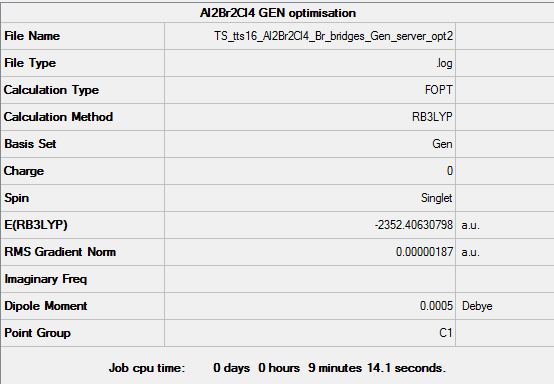
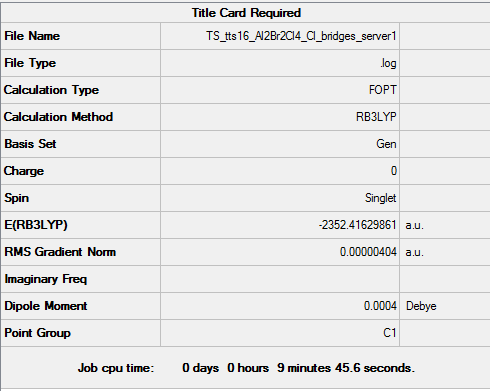
Al2Br2Cl4 with bromine bridges
Key data for Al2Br2Cl4 (bromine bridges)
For the following optimisation, pseudo-potentials and basis sets were both used under the GEN function setting. Aluminium and chlorine atoms were optimised with B3LYP/6-31G(d,p), while Br was optimized with LanL2DZ pseudo-potentials as it is a much heavier atom.
Energy of this isomer = -2352.40631 a.u. = -6175067 kJ/mol
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000040 0.001800 YES
RMS Displacement 0.000015 0.001200 YES
Low frequencies --- -5.1748 -5.0356 -3.1468 -0.0015 -0.0012 -0.0011 Low frequencies --- 14.8260 63.2702 86.0770
Link to File:TS TTS16 AL2BR2CL4 BR BRIDGES GEN SERVER FREQ2.LOG
Al2Br2Cl4 (Br bridges) molecule |
Al2Br2Cl4 with chlorine bridges
Key data for Al2Br2Cl4 (chlorine bridges)
For the following optimisation, pseudo-potentials and basis sets were both used under the GEN function setting. Aluminium and chlorine atoms were optimised with B3LYP/6-31G(d,p), while Br was optimized with LanL2DZ pseudo-potentials as it is a much heavier atom.
Energy of this isomer = -2352.41630 a.u. = -6175093 kJ/mol
Ng611 (talk) 18:34, 21 May 2018 (BST) You were ever so slightly off with your energy here.
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000054 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Low frequencies --- -5.1490 0.0032 0.0032 0.0040 1.4146 2.0504 Low frequencies --- 18.1472 49.1065 73.0086
Link to File:TS TTS16 AL2BR2CL4 CL BRIDGES SERVER FREQ1.LOG
Al2Br2Cl4 (Cl bridges) molecule |
The energies of the bridging bromine isomer and the bridging chlorine isomer are -6175067 and -6175093 kJ/mol respectively, indicating that the bridging chlorine isomer is one of higher stability. The higher stability of the chlorine bridging system arises from the better overlap of the chlorine and aluminium orbitals. Aluminium and chlorine are both in the 3rd row of the periodic table, while bromine is in the fourth row. Consequently, the orbitals of aluminium and chlorine are more comparable in both energy and size thus giving a more favorable interaction.
Ng611 (talk) 18:27, 21 May 2018 (BST) Your stability values are correct, as is your rationalisation - well done. I would suggest calculating the energy difference as opposed to reporting the raw values though as it makes your point a bit clearer.
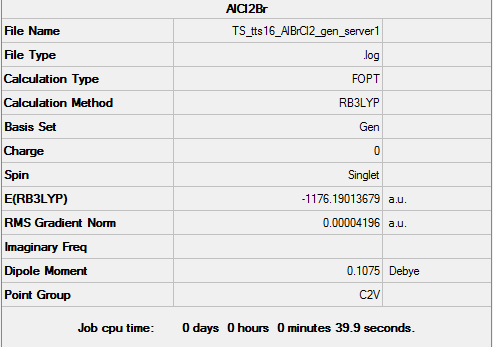
AlBrCl2 Monomer
Key data for monomer AlBrCl2
For the following optimisation, pseudo-potentials and basis sets were both used under the GEN function setting. Aluminium and chlorine atoms were optimised with B3LYP/6-31G(d,p), while Br was optimized with LanL2DZ pseudo-potentials as it is a much heavier atom.
Energy of monomerː -1176.19014 a.u. = -3087499 kj/mol
Item Value Threshold Converged?
Maximum Force 0.000136 0.000450 YES
RMS Force 0.000073 0.000300 YES
Maximum Displacement 0.000681 0.001800 YES
RMS Displacement 0.000497 0.001200 YES
Low frequencies --- -0.0032 -0.0027 0.0015 1.3569 3.6367 4.2604 Low frequencies --- 120.5042 133.9178 185.8950
Link to File:TS TTS16 ALBRCL2 GEN SERVER FREQ.LOG
AlBrCl2 monomer |
Below are the energies of the dimer and monomer of AlBrCl2.
Energy of Al2Br2Cl4 chlorine bridging isomer = -6175093 kJ/mol
Energy of AlBrCl2 monomer = -3087499 kJ/mol
Dissociation energy = [2 x E(Monomer)] - E(Isomer) = 95 kJ/mol
Ng611 (talk) 18:36, 21 May 2018 (BST) Correct sign, but your value is off by 60 kJ/mol - likely due to an incorrect conversion between hartrees and Kj/mol.
The dissociation energy of the isomer to monomer is ̟95 kj/mol, thus AlBrCl2 exists as a Al2Br2Cl4 dimer as it is more stable relative to its monomer. The dimer is more stable as dimerisation helps to relieve the electron deficiency of the aluminum atoms through the halogen bridges.
Ng611 (talk) 18:37, 21 May 2018 (BST) Well explained!
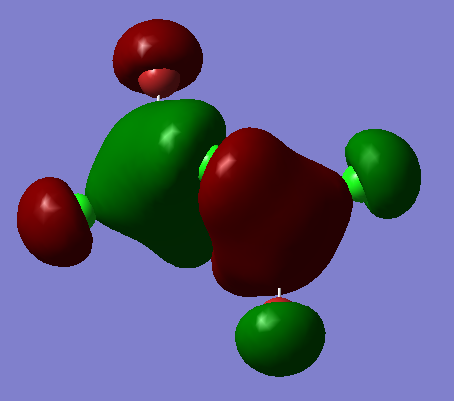
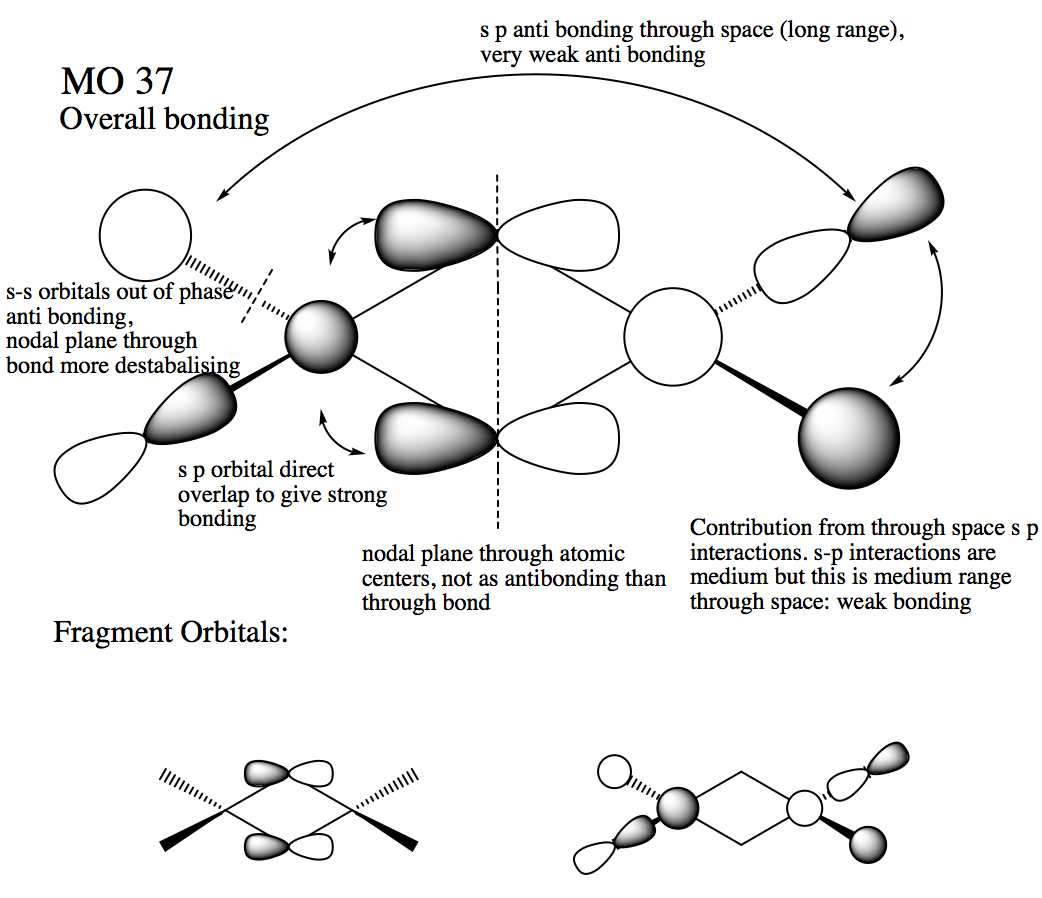
Visualising molecular orbitals of the Al2Br2Cl4 chlorine bridging isomer
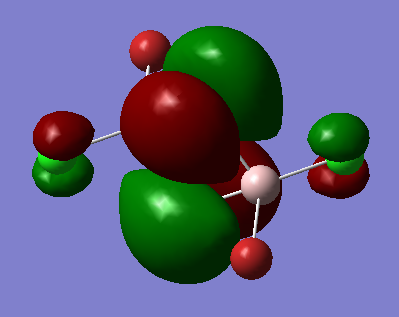
Molecular orbital 37
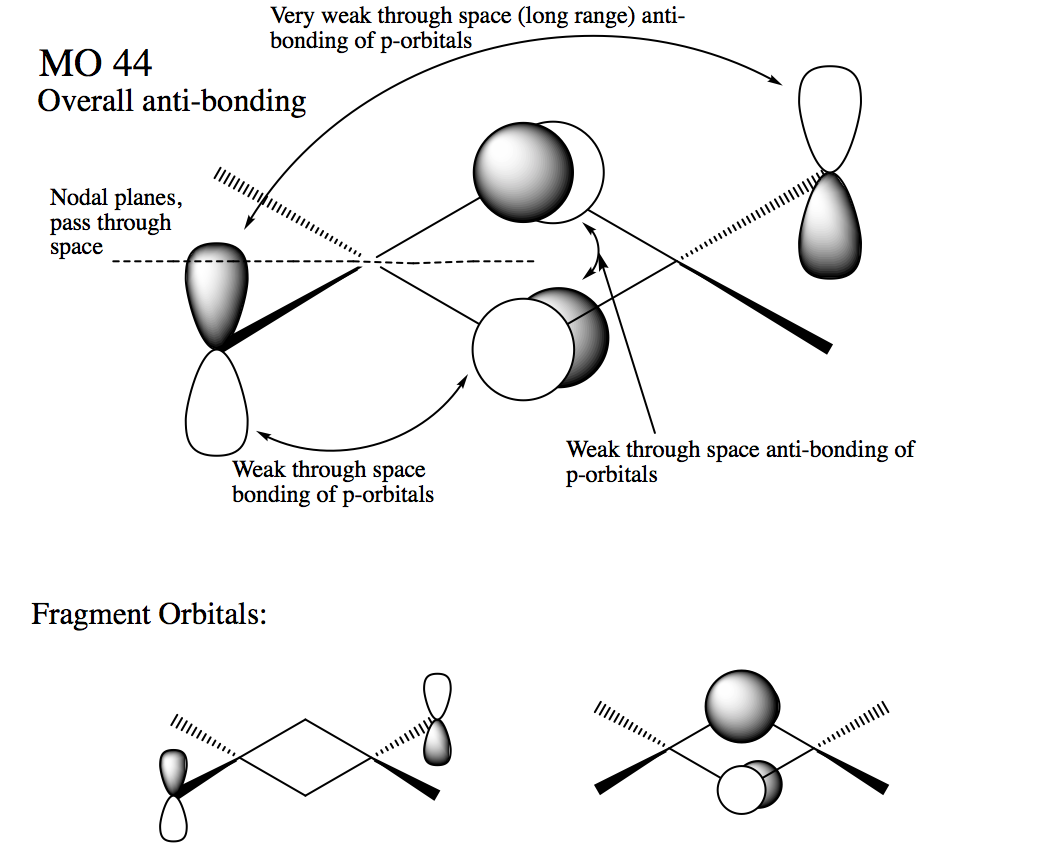
Ng611 (talk) 18:41, 21 May 2018 (BST) Not sure I agree with you 100% on your LCAO decomposition -- the orbitals on the terminal halides look more like s-orbitals polarised off the atoms rather than a mix of s and p orbitals.
Molecular orbital Molecular orbital 44
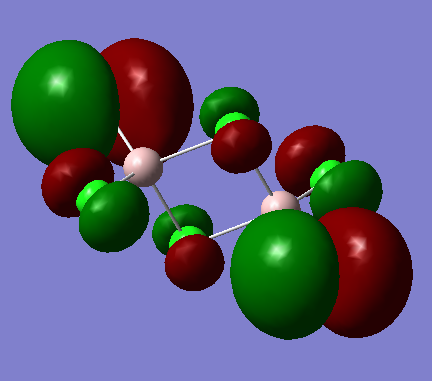
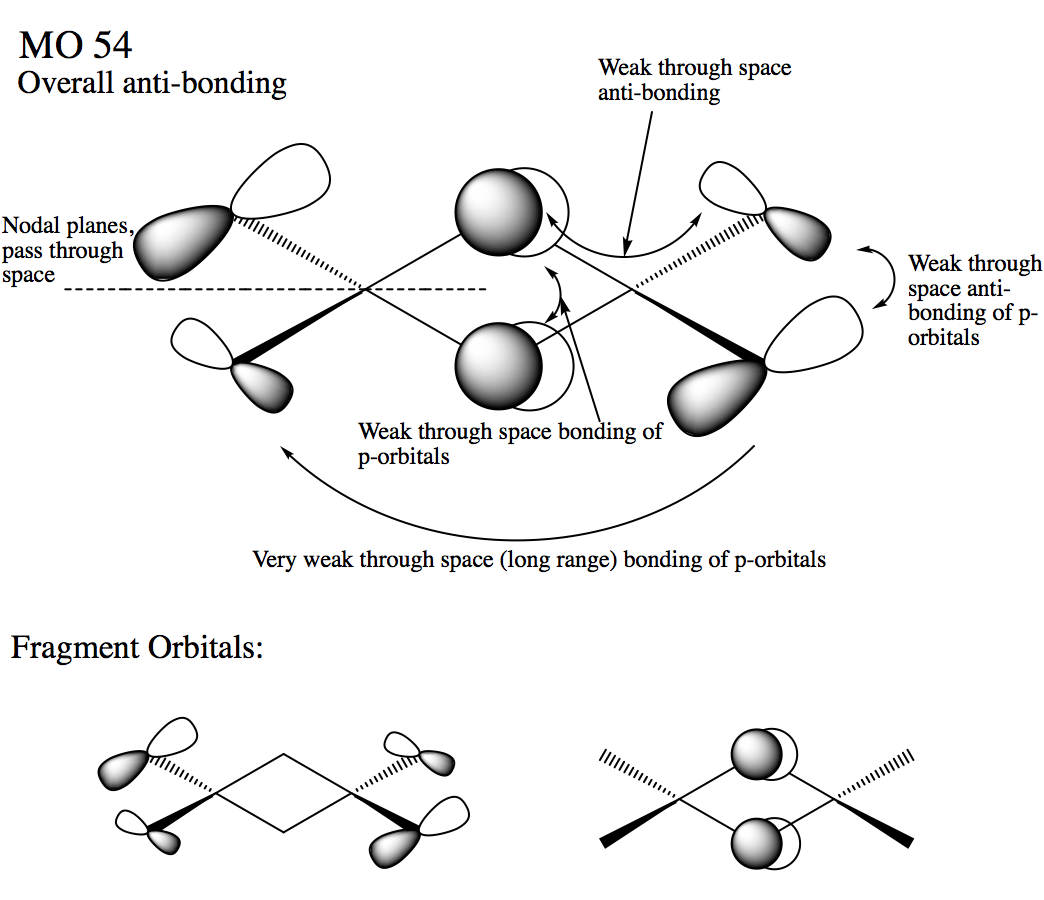
Molecular orbital 54
Ng611 (talk) 18:47, 21 May 2018 (BST) A very good report, well done. Be careful with your LCAO analysis though - sometimes s orbitals may be polarised off the atom and look instead like p-orbitals. Otherwise, there were no major issues; this was a very good report.