Rep:Mod:tb1217
NH3
Summary
NH3, commonly referred to as Ammonia is a colourless gas with a pungent odour[1]
The molecule was optimised using GaussView with the following criteria:
| Calculation Method | B3LYP |
| Basis Set | 6-31G(d,p) |
Optimisation was carried out to calculate the values for the molecule with the optimum position of the nuclei for the electron configuration of the molecule.
Having optimised the molecule, the following values were calculated:
| Value | |
|---|---|
| E(RB3LYP) | -56.5577687 AU |
| RMS gradient | 0.00000485 AU |
| Point Group | C3v |
| Bond angle H-N-H | 105.7° |
| Bond distance H-N | 1.02 Å |
The angles have been calculated with an accuracy of 1° and the distance with 0.01Å
Item section of the output:
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000070 0.001800 YES
RMS Displacement 0.000033 0.001200 YES
Predicted change in Energy=-5.785197D-10
Optimization completed.
-- Stationary point found.
Viewing NH3
NH3 |
Vibrations of NH3
| Value | ||||
|---|---|---|---|---|
| Wavenumber (cm-1) | 1090 | 1694 | 3460 | 3590 |
| Symmetry | A1 | E | A1 | E |
| Intensity (arbitrary units) | 145 | 13.6 | 1.06 | 0.27 |
| Image |  |
 |
 |

|

Using the 3N-6 rule, NH3, with N=4, is expected to have 6 modes of vibration. It has 2 degenerate modes of vibration, 2 with an intensity of 13.6, and 2 with an intensity of 0.27. An IR spectrum has peaks when there is a change in dipole moment for the bonds, where the intensity listed above corresponds to the height of the peak on the spectrum. On the IR spectrum of NH3, 2 bands are expected; one for 1090cm-1 and one for 1694cm-1. The bands for 3460cm-1 and 3590cm-1 are not seen due to there being no overall change in dipole moment. The vibrations of 1090cm-1 and 1694cm-1 vibrations are bending. The vibrations of 3460cm-1 and 3590cm-1 are bond stretching vibrations. The modes for 1090cm-1 and 3460cm-1 are both symmetric, where the mode 3460cm-1 mode is highly symmetric as the point group does not move as it does in the mode for 1090-1.
Additionally, the 1090cm-1 mode is known as the umbrella mode due to the way in which the bonds bend.
Charge Analysis of NH3

Nitrogen is more electronegative than Hydrogen, hence has a negative charge and Hydrogen has a positive charge. As Nitrogen is more electronegative, it has a greater pull on the shared pair of electrons hence has a negative charge.
N2
Summary
N2 is a 'colourless, odourless, tasteless' gas and is needed for many biological processes as well as in the production of fertiliser [2]
The molecule was optimised using GaussView with the following criteria:
| Calculation Method | B3LYP |
| Basis Set | 6-31G(d,p) |
Optimisation was carried out to calculate the values for the molecule with the optimum position of the nuclei for the electron configuration of the molecule.
Having optimised the molecule, the following values were calculated:
| Value | |
|---|---|
| E(RB3LYP) | -109.5241287 AU |
| RMS gradient | 0.0000006 AU |
| Point Group | D∞h |
| Bond distance H-N | 1.11 Å |
The angles have been calculated with an accuracy of 1° and the distance with 0.01Å
Item section of the output:
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.383662D-13 Optimization completed.
Viewing N2
N2 |
Vibrations of N2
| Value | |
|---|---|
| Wavenumber (cm-1) | 2457 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0 |
| Image | 
|
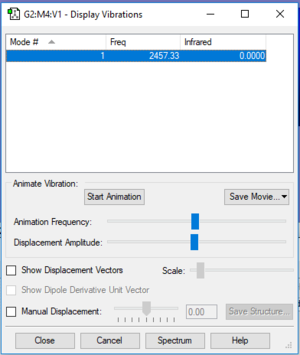
The IR spectrum of N2 has no peaks due as the single vibrational mode that does occur, bond stretching, has no overall change in dipole moment. Hence, there is no peak for the mode which occurs at 2457cm-1.
Charge Analysis of N2

The molecule is made up of 2 Nitrogen atoms, hence the individual atoms do not have a charge. Furthermore, the molecule itself also does not have a charge.
Mono-metallic TM complex of N2
TRANS-BIS(1,1'-BIS(DIETHYLPHOSPHINO)FERROCENE)-bis(dinitrogen) (structure identifier AGISEP) has a crystal structure and contains a dinitrogen bond of length of 1.14Å. This is longer than the computed length of 1.11Å.
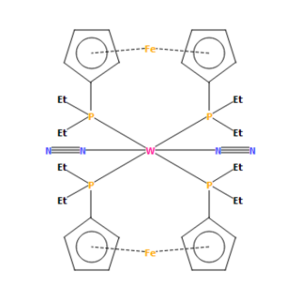
The crystal structure has a longer bond length for the dinitrogen triple bond than the value computed. One of the factors as to why this occurs is due to the Tungsten atom which, due to its electronegativity, pulls electron density away from the Nitrogen involved in the N2 bond. This reduces the bond strength and also lengthens the bond, hence making it longer than the computed length of 1.11Å.
Link to structure: https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=AGISEP&DatabaseToSearch=Published
H2
Summary
H2 is also a colourless, tasteless, and odourless gas and is also extremely flammable [3].
The molecule was optimised using GaussView with the following criteria:
| Calculation Method | B3LYP |
| Basis Set | 6-31G(d,p) |
Optimisation was carried out to calculate the values for the molecule with the optimum position of the nuclei for the electron configuration of the molecule.
Having optimised the molecule, the following values were calculated:
| Value | |
|---|---|
| E(RB3LYP) | -1.1785394 AU |
| RMS gradient | 0.00000017 AU |
| Point Group | D∞h |
| Bond distance H-N | 0.74 Å |
The angles have been calculated with an accuracy of 1° and the distance with 0.01Å
Item section of the output:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.167770D-13 Optimization completed.
Viewing H2
H2 |
Vibrations of H2
| Value | |
|---|---|
| Wavenumber (cm-1) | 4466 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0 |
| Image | 
|
Similarly to N2, the IR spectrum of H2 has no peaks as the single vibrational mode that does occur, bond stretching, has no overall change in dipole moment. Hence, there is no peak for the mode which occurs at 4466cm-1.
Charge Analysis of H2
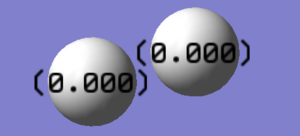
The molecule is made up of 2 Hydrogen atoms, hence the individual atoms do not have a charge. Furthermore, the molecule itself also does not have a charge.
Haber-Bosch Process
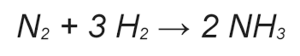
The Haber-Bosch Process is the reaction used to produce fertiliser. N2 and H2 gases react to form ammonia. In the following table, the energies of the reaction has been given:
| Energy (au) | |
|---|---|
| E(NH3) | -56.5577687 |
| 2*E(NH3) | -113.1155374 |
| E(N2) | -109.5241287 |
| E(H2) | -1.1785394 |
| 3*E(H2) | -3.5356182 |
The total energy for the reaction is 2*E(NH3) - [E(N2)+3*E(H2)] and using the values from above, is calculated as -0.0557905au. Energy differences are reported in kJmol-1 and for this reaction is -146.476645kJmol-1, -146.5kJmol-1 (1dp). The reaction has a negative energy change, hence the reaction is exothermic and the ammonia product is more stable than the gaseous N2 and H2 as it is of a lower energy than the reactants.
PF5
Summary
PF5, phosphorus pentafluoride, is a colourless and toxic gas which adopts a trigonal bipyramidal geometry.
The molecule was optimised using GaussView with the following criteria:
| Calculation Method | B3LYP |
| Basis Set | 6-31G(d,p) |
Optimisation was carried out to calculate the values for the molecule with the optimum position of the nuclei for the electron configuration of the molecule.
Having optimised the molecule, the following values were calculated:
| Value | |
|---|---|
| E(RB3LYP) | -840.6763460 AU |
| RMS gradient | 0.01907AU |
| Point Group | C3v |
| Bond angle F-P-F | 90° and 120° |
| Bond distance P-F | 1.57 Å |
The angles have been calculated with an accuracy of 1° and the distance with 0.01Å
Item section of the output:
Item Value Threshold Converged?
Maximum Force 0.000299 0.000450 YES
RMS Force 0.000090 0.000300 YES
Maximum Displacement 0.000867 0.001800 YES
RMS Displacement 0.000268 0.001200 YES
Predicted change in Energy=-2.765727D-07
Optimization completed.
-- Stationary point found.
Viewing PF5
PF5 |
Vibrations of PF5
| Value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Wavenumber (cm-1) | 172 | 478 | 503 | 544 | 669 | 784 | 997 | 1020 |
| Symmetry | E' | E | E' | A2 | A1' | A1' | A2 | E' |
| Intensity (arbitrary units) | 0.035 | 0 | 37.9 | 47.3 | 0 | 0 | 363 | 248 |
| Image |  |
 |
 |
 |
 |
 |

| |
The IR spectrum of PF5 has 4 peaks at 503cm-1, 544cm-1, 997cm-1, and 1020cm-1.
Charge Analysis of PF5
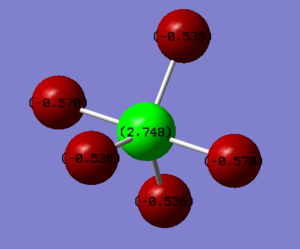
Fluorine is more electronegative than Phosphorus, hence has a negative charge and Phosphorus has a positive charge. As Fluorine is more electronegative, it has a greater pull on the shared pair of electrons hence has a negative charge. There are two different charges for the Fluorine; -0.570 and -0.536. Due to the geometry of the molecule, the Fluorine atoms vary by charge depending on their position in the trigonal bipyramidal shape.
Molecular Orbitals of PF5
Below is a table of several occupied molecular orbitals (MO) of PF5 computed using Gaussview.
HCN
Summary
HCN, Hydrogen Cyanide, is an extremely flammable and also poisonous substance which exists at room temperature as a liquid. There is a single bond between Hydrogen and the central Carbon, and a triple bond between the Nitrogen and central Carbon. Overall, the molecule adopts a linear geometry.
The molecule was optimised using GaussView with the following criteria:
| Calculation Method | B3LYP |
| Basis Set | 6-31G(d,p) |
Optimisation was carried out to calculate the values for the molecule with the optimum position of the nuclei for the electron configuration of the molecule.
Having optimised the molecule, the following values were calculated:
| Value | |
|---|---|
| E(RB3LYP) | -93.4245813AU |
| RMS gradient | 0.00017006AU |
| Point Group | C1 |
| Bond distance C-H | 1.07 Å |
| Bond distance C-N (triple bond) | 1.16 Å |
The angles have been calculated with an accuracy of 1° and the distance with 0.01Å
Item section of the output:
Item Value Threshold Converged?
Maximum Force 0.000370 0.000450 YES
RMS Force 0.000255 0.000300 YES
Maximum Displacement 0.000731 0.001800 YES
RMS Displacement 0.000459 0.001200 YES
Predicted change in Energy=-2.199408D-07
Optimization completed.
-- Stationary point found.
Viewing HCN
HCN |
References
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES - overall your wiki is very good well done! Excellent explanations particularly for the first part.
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES, good explanation on the charges. The MOs are nicely presented and most of your written explanations are correct, well done. However you have missed out explaining whether interactions are bonding or anti-bonding. For example MO 11 and MO 16 are formed of in phase (same colour, bonding, MO11) and out of phase (different colour, antibonding, MO 16) AOs. You will develop confidence with this concept over your upcoming lecture courses, don't worry!
Independence 1/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
You added lots of extra references, well done.
Do an extra calculation on another small molecule, or
You also calculated HCN, good work.
Do some deeper analysis on your results so far