Rep:Mod:taxol
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
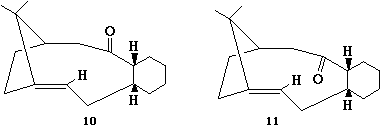
A key intermediate 10 or 11 in the total synthesis of Taxol (an important drug in the treatment of ovarian cancers) proposed by Paquette is initially synthesised with the carbonyl group pointing either up or down. On standing, the compound apparently isomerises to the alternative carbonyl isomer. This is an example of atropisomerism. Clearly the stereochemistry of carbonyl addition depends on which isomer is the most stable. It is also noted that during subsequent functionalisation of the alkene, this reacted abnormally slowly!
Procedure
Using molecular mechanics (MM2) to determine the most stable isomer 10 or 11, and to rationalise why the alkene reacts slowly (hint: find literature on hyperstable alkenes!). Pay particular attention to the conformation of the resulting optimised structure, to see if any aspect of this structure could be improved by further minimisations (preceeded if necessary by a manual edit of the structure to move atoms into more correct orientations).
Estimated time for completion: < 30 minutes per isomer (< 1 hour in total).
Key literature
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466 (DOI:10.1021/ja00824a025 DOI:10.1021/ja00824a025 ) and 95, 3051 for another nice example of atropisomerism.
- Another well known example is within Vancomycin: J. Am. Chem. Soc., 1999, 121, 3226. DOI: 10.1021/ja990189i
- An interesting variation is of "atropenantioselective cycloetherification" (G.ÊIslas-Gonzalez, M.ÊBois-Choussy and J.ÊZhu, Org. Biomol. Chem., 2003, 30-32. DOI: 10.1039/b208905. See also Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI: 10.1016/j.tetasy.2004.11.004
