Rep:Mod:takasugi
Wang Simeng
Year 3 Computational Lab
Module 3
Transition State
Tutorial: Cope Rearrangement 1, Optimisation of Reactants and Products
Optimisation of anti 1,5-hexandiene
anti 4 hexadiene |
Set-up
- Method: HF
- Basis set: 3-21G
- Job Type: Optimisation
- Keywords: # opt HF/3-21g geom=connectivity
Results
The log file of anti 1,5-hexadiene (opt 3-21G) is available here.
- Summary Table
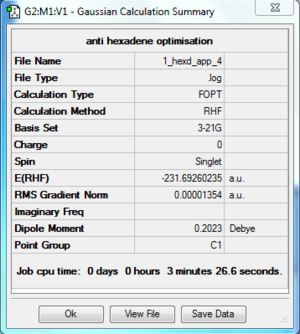
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.69260235 a.u. |
| Gradient | 0.00001354 a.u. |
| Dipole Moment | 0.2023 Debye |
| Point Group | C2 |
| Calculation Time | 3 min 26.6 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000019 0.000450 YES
RMS Force 0.000008 0.000300 YES
Maximum Displacement 0.000710 0.001800 YES
RMS Displacement 0.000210 0.001200 YES
Predicted change in Energy=-2.165407D-08
Optimization completed.
-- Stationary point found.
Both the forces and the placements are converged.
Optimisation of gauche 1,5-hexandiene
gauche 1 hexadiene |
Set-up
- Method: HF
- Basis set: 3-21G
- Job Type: Optimisation
- Keywords: # opt HF/3-21g geom=connectivity
Results
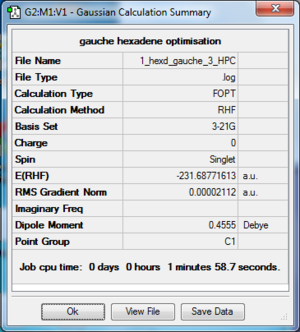
The log file of gauche 1,5-hexadiene (opt 3-21G) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.68771613 a.u. |
| Gradient | 0.00002112 a.u. |
| Dipole Moment | 0.2023 Debye |
| Point Group | C2 |
| Calculation Time | 1 min 58.7 s |
- Item Table
Item Value Threshold Converged? Maximum Force 0.000042 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000885 0.001800 YES RMS Displacement 0.000277 0.001200 YES Predicted change in Energy=-5.214105D-08 Optimization completed. -- Stationary point found.
Discussion
Both app and gauche conformer obtained have C2 point groups. However, the gauche conformer has a higher energy than the app conformer. The gauche form was 0.004 a.u.(3.066 kcal/mol) higher in energy. This is expected as the gauche conformation obtained in this section was slightly more crowded than other possible gauche conformers. The electron rich double bonds are pointing to the same point and are close to each other. There may be a higher extent of repulsion than other possible gauche conformers. The anti-periplanar conformer generally less likely to have high electrostatic repulsion as the larger groups are 180 degrees away from each other.
Optimisation of the lowest energy conformation of 1,5-hexandiene
In normal cases, app conformers tends to have lower energy for hydrocarbon chains as it tends to reduce electrostatic repulsion of the carbon chain by placing the 2 larger substituents away from each other. Hence, it is postulated that the app conformer found in the previous session has the lowest energy.
However, the longer the chain is, there are more bonds with free rotation. As a result, the number of distinct conformers increase and there are more factors to affect the energy. it was found that one of the gauche conformer is slightly lower in energy (by 0.04 a.u.) than the app conformer found earlier. This is a minimal difference, which might be caused by factor other than steric hinderance. The presence of double bond on both side might be responsible for app conformers having higher energy than this particular gauche conform.
gauche 3 hexadiene |
Set-up
- Method: HF
- Basis set: 3-21G
- Job Type: Optimisation
- Keywords: # opt HF/3-21g geom=connectivity
Results
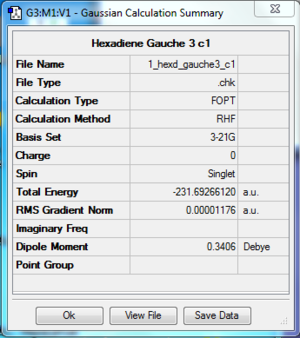
The log file of postulated lowest energy conformation 1,5-hexadiene (opt 3-21G) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.69266120 a.u. |
| Gradient | 0.00001176 a.u. |
| Dipole Moment | 0.3406 Debye |
| Point Group | C1 |
| Calculation Time | 36.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000018 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.001003 0.001800 YES
RMS Displacement 0.000326 0.001200 YES
Predicted change in Energy=-2.568608D-08
Optimization completed.
-- Stationary point found.
Discussion
Enthalpy Change
Activation Energy
Optimisation of the anti 2 conformer of 1,5-hexandiene
anti 2 Ci hexadiene |
Set-up
- Method: HF
- Basis set: 3-21G
- Job Type: Optimisation
- Keywords: # opt HF/3-21g geom=connectivity
Results
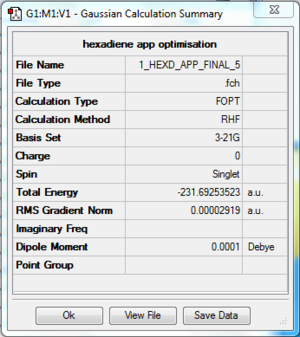
The log file of anti 2 conformer 1,5-hexadiene (opt 3-21G) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.69253523 a.u. |
| Gradient | 0.00002919 a.u. |
| Dipole Moment | 0.0001 Debye |
| Point Group | Ci |
| Calculation Time | 1 min 2.7 s |
The energy obtained was the same as shown in the table of low energy conformer.
E(calculated) =-231.69253523 a.u. E(conformer table) =-231.69254 a.u.
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000123 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.001272 0.001800 YES
RMS Displacement 0.000316 0.001200 YES
Predicted change in Energy=-7.437503D-08
Optimization completed.
-- Stationary point found.
Optimisation of the anti 2 conformer at the B3LYP/6-31G* Level
Set-up
- Method: B3LYP
- Basis set: 6-31G*
- Job Type: Optimisation
- Keywords: # opt b3lyp/6-31g(d) geom=connectivity
Results
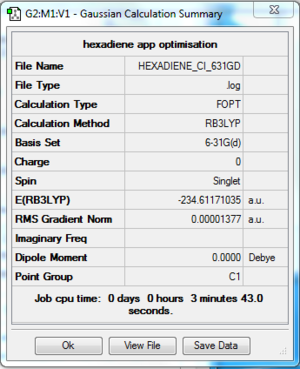
The log file of anti 2 conformer 1,5-hexadiene (opt 6-31G*) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Final Energy | -234.61171035 a.u. |
| Gradient | 0.00001377 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | Ci |
| Calculation Time | 3 min 43.0 s |
After optimisation at 6-31G* level, the energy of the molecule is further lowered. However, the symmetry of the molecule remains the same as Ci and not much visible change in the structure of the molecule was observed.
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000015 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000269 0.001800 YES
RMS Displacement 0.000091 0.001200 YES
Predicted change in Energy=-1.692985D-08
Optimization completed.
-- Stationary point found.
Frequency of the anti 2 conformer at the B3LYP/6-31G* Level
Set-up
- Method: B3LYP
- Basis set: 6-31G*
- Job Type: Frequency
- Keywords: # freq b3lyp/6-31g(d) geom=connectivity
Results
The log file of anti 2 conformer 1,5-hexadiene (opt 6-31G*) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Final Energy | -234.61171035 a.u. |
| Gradient | 0.00001376 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | Ci |
| Calculation Time | 3 min 2.0 s |
- Low Frequencies Table
Low frequencies --- -9.4816 -0.0011 -0.0006 -0.0004 3.7607 13.0292 Low frequencies --- 74.2876 80.9991 121.4193
Discussion
- Temperature Corrections
Zero-point correction= 0.142507 (Hartree/Particle) Thermal correction to Energy= 0.149853 Thermal correction to Enthalpy= 0.150797 Thermal correction to Gibbs Free Energy= 0.110933 Sum of electronic and zero-point Energies= -234.469204 Sum of electronic and thermal Energies= -234.461857 Sum of electronic and thermal Enthalpies= -234.460913 Sum of electronic and thermal Free Energies= -234.500777
Tutorial: Cope Rearrangement 2, Optimisation of Transition state
Optimisation of Allyl Fragment
Allyl Fragment |
Set-up
- Method: HF
- Basis set: 3-21G
- Job Type: Optimisation
- Keywords: # opt HF/3-21g geom=connectivity
Results
The log file of the optimised allyl fragment (opt 3-21G) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -115.82303997 a.u. |
| Gradient | 0.00007210 a.u. |
| Dipole Moment | 0.0291 Debye |
| Point Group | C2v |
| Calculation Time | 0 min 12.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000160 0.000450 YES
RMS Force 0.000051 0.000300 YES
Maximum Displacement 0.000990 0.001800 YES
RMS Displacement 0.000359 0.001200 YES
Predicted change in Energy=-1.268189D-07
Optimization completed.
-- Stationary point found.
Both the forces and the placements are converged.
Optimisation of chair transition state
Chair Transition State |
Set-up
- Method: HF
- Basis set: 3-21G
- Job Type: Opt+Freq (to TS(Berny))
- Additional Keywords: Opt=NoEigen
- Keywords: # opt=(calcfc,ts,noeigen) freq hf/3-21g geom=connectivity
Results
The log file of chair transition state (opt 3-21G) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.61932238 a.u. |
| Gradient | 0.00002235 a.u. |
| Dipole Moment | 0.0005 Debye |
| Point Group | C2h |
| Calculation Time | 10.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.001564 0.001800 YES
RMS Displacement 0.000263 0.001200 YES
Predicted change in Energy=-9.355219D-08
Optimization completed.
-- Stationary point found.
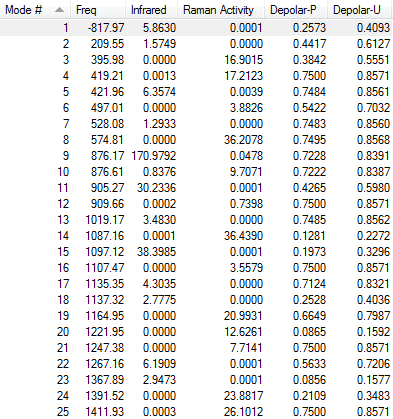
Low frequencies --- -817.9694 -2.5286 0.0003 0.0006 0.0008 1.9022
Low frequencies --- 3.0705 209.5461 395.9801
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -817.9694 209.5461 395.9801
The calculation was converged and the optimisation was successful.
1 imaginary frequency is detected (-817.9694 cm-1), showing that it is a transition state.
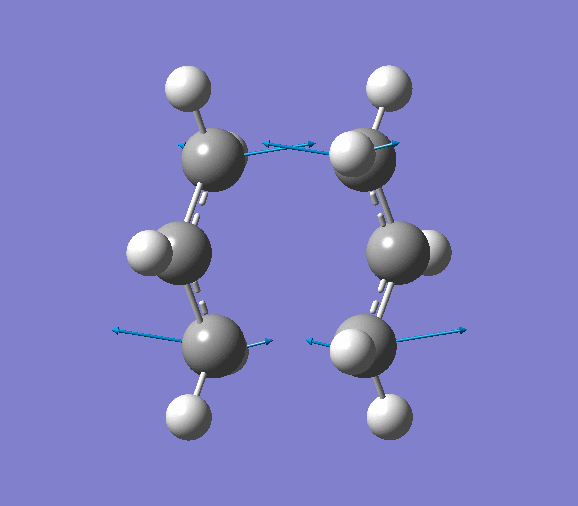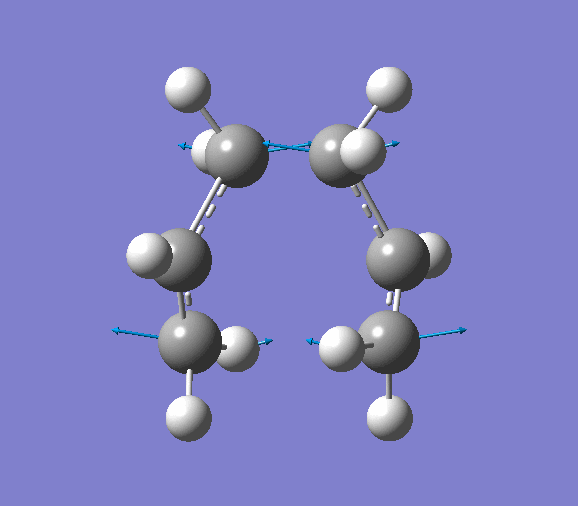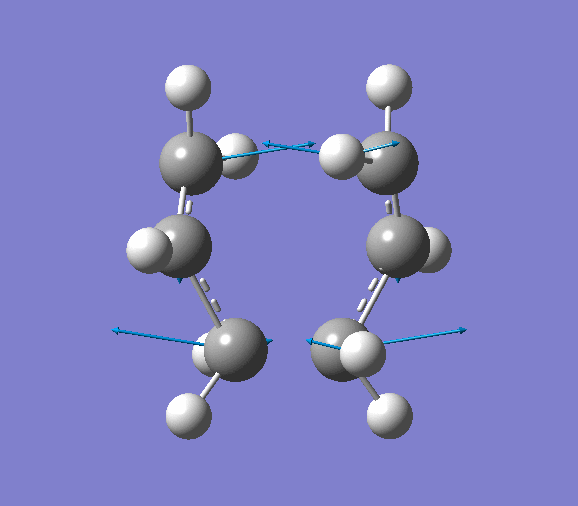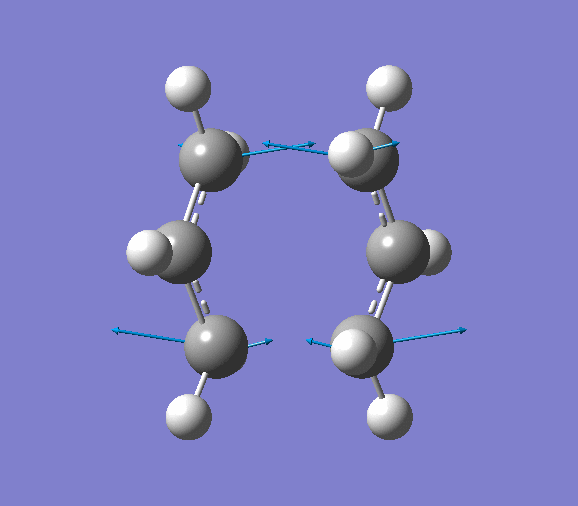
- Modes of Vibration
The vibration of the imaginary frequency is shown below (animation is available on the right). It can be seen that the movements of the terminal carbon atoms are sychronised in 2 groups. The terminal C atoms in each fragment come closer which facilitates bond forming while the pair of terminal carbons on the opposite side move away from each other, signifies breaking a bond. This is what is observed in Cope rearrangement, whereby one C-C bond is broken and one is formed in a 6-membered ring like system.
Optimisation of chair Transition State using Redundant Coordinate Editor
In this section, the molecule is optimised using Redundant Coord Editor. In the first part, the molecule was optimised while the coordinate of the atoms forming the new bonds are frozen. In the second part, the new bond is then optimised.
However, due to the recent update of Gaussview, the syntax representing frozen coordinate is no longer recognised by the program and this operation has become meaningless. In the result, it was shown that both the structure and the new bond were optimised directly in the first part. Due to this reason, both parts have the same results.
Set-up 1: Freeze Coordinate
- Method: HF
- Basis set: 3-21G
- Job Type: Opt+Freq (to TS(Berny))
- Additional Keywords: Opt=NoEigen
- Keywords: # opt=(calcfc,ts,modredundant,noeigen) freq hf/3-21g geom=connectivity
Results 1: Freeze Coordinate
The log file of chair transition state using RCE (freezing bond) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.61932245 a.u. |
| Gradient | 0.00001430 a.u. |
| Dipole Moment | 0.0001 Debye |
| Point Group | C2h |
| Calculation Time | 9.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000715 0.001800 YES
RMS Displacement 0.000123 0.001200 YES
Predicted change in Energy=-2.789741D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -817.9062 -2.7274 -0.4687 0.0008 0.0009 0.0010
Low frequencies --- 2.1409 209.5426 395.9879
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -817.9062 209.5426 395.9879
Set-up 2: Derivative
- Method: HF
- Basis set: 3-21G
- Job Type: Opt+Freq (to TS(Berny))
- Additional Keywords: Opt=NoEigen
- Keywords: # opt=(ts,modredundant,noeigen) freq hf/3-21g geom=connectivity
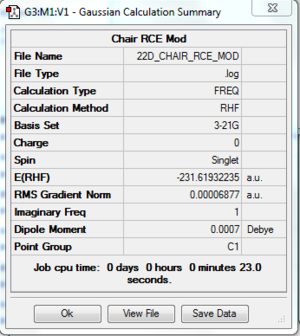
Results 2: Derivative
The log file of chair transition state using RCE (optimising bond) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.61932235 a.u. |
| Gradient | 0.00006877 a.u. |
| Dipole Moment | 0.0007 Debye |
| Point Group | C2h |
| Calculation Time | 23.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000025 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000328 0.001800 YES
RMS Displacement 0.000078 0.001200 YES
Predicted change in Energy=-1.621693D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -817.8260 -1.1023 0.0004 0.0008 0.0009 1.9363
Low frequencies --- 7.9385 209.8904 396.3304
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -817.8260 209.8902 396.3304
Discussion
|
Optimisation Method |
Bond length of the new bond /Å | Bond length of other bonds /Å |
|---|---|---|
| HF/3-21 | 2.02050
2.02030 |
1.38927
1.38928 |
| HF/3-21
using RCE |
2.01951
2.02038 |
1.38931
1.38920 |
The same final energy was obtained by using both ways to optimise the chair conformer. The bond length obtained was very similar as well. The difference was within 0.001 Å.
Unfortunately the RCE is not working with the new version of gaussview. From the first step of the RCE optimisation, the same result as using HF/3-21G directly was obtained. Hence, it is not meaningful to discuss further about any difference may occur between these 2 sets of the results.
Optimisation of boat Transition State using QST2
boat transition state |
The boat transition state was obtained by optimise the anti 2 (Ci) conformer of hexadiene using QST2. However, the structure of the anti 2 conformer has to be tuned to a structure similar to the transition state, in order for the calculation to work. In the first part, the calculation was done on the original anti 2 conformer and the calculation failed. A chair looking structure was obtained. In the second part, the bond angle was changed to give a ring like structure, and a transition structure with C2v symmetry was obtained.
Set-up 1: Original anti 2 Conformer
- Method: HF
- Basis set: 3-21G
- Job Type: Opt+Freq (to TS(QST2))
- Keywords: # opt=qst2 freq hf/3-21g geom=connectivity
Results: Original anti 2 Conformer
The log file of boat transition state (qst2) is available here.
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | FOPT |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.62383534 a.u. |
| Gradient | 0.01454126 a.u. |
| Dipole Moment | 0.0960 Debye |
| Point Group | C2 |
| Calculation Time | 28.0 s |
- Error Message
Item Value Threshold Converged? Maximum Force 0.024802 0.000450 NO RMS Force 0.007566 0.000300 NO Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-5.113754D-17 Optimization aborted. -- No acceptable step. Error termination request processed by link 9999. Error termination via Lnk1e in C:\G09W\l9999.exe at Thu Oct 25 13:09:45 2012.
The calculation was ended with an error message probably because the structure submitted was not close enough to the transition state. The result obtained is shown below and it is very different from the real transition state. The arrangement is in fact closer to the chair conformer.
boat transition state fail |
Set-up 2: Ring Structure
- Method: HF
- Basis set: 3-21G
- Job Type: Opt+Freq (to TS(QST2))
- Keywords: # opt=qst2 freq hf/3-21g geom=connectivity
Results: Ring Structure
The log file of boat transition state (qst2) is available here.
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RHF |
| Basis Set | 3-21G |
| Final Energy | -231.60280218 a.u. |
| Gradient | 0.00009221 a.u. |
| Dipole Moment | 0.1578 Debye |
| Point Group | C2v |
| Calculation Time | 17.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000132 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.001253 0.001800 YES
RMS Displacement 0.000270 0.001200 YES
Predicted change in Energy=-2.856083D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -839.6188 -2.4561 0.0007 0.0008 0.0010 4.8228
Low frequencies --- 7.6438 155.4889 381.7658
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -839.6188 155.4882 381.7658
Optimisation at 6-31G* Level
Set-up
- Method: B3LYP
- Basis set: 6-31G*
- Job Type: Opt+Freq (to TS(Berny))
- Keywords: # opt=(calcfc,ts) freq b3lyp/6-31g(d) geom=connectivity
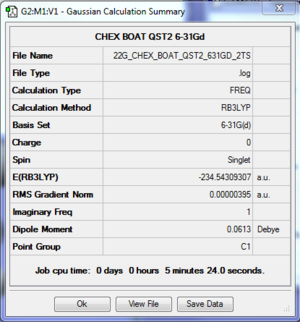
Results: Optimisation of Boat Conformer
Boat Conformer 6-31* |
The log file of boat transition state (B3LYP/6-31*) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Final Energy | -234.54309307 a.u. |
| Gradient | 0.00000395 a.u. |
| Dipole Moment | 0.0613 Debye |
| Point Group | C2v |
| Calculation Time | 5 min 24.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000009 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000147 0.001800 YES
RMS Displacement 0.000051 0.001200 YES
Predicted change in Energy=-2.569998D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -530.3629 -8.3995 0.0004 0.0007 0.0008 15.4646
Low frequencies --- 17.6156 135.6115 261.6996
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -530.3629 135.5557 261.6996
The calculation was converged and the optimisation was successful.
There was 1 imaginary frequency observed, indicating that it is a transition state.
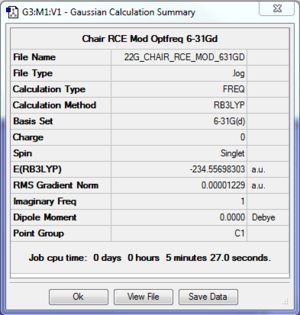
Results: Optimisation of Chair Conformer
Chair Conformer 6-31* |
The log file of chair transition state (B3LYP/6-31*) is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Final Energy | -234.55698303 a.u. |
| Gradient | 0.00001229 a.u. |
| Dipole Moment | 0.0000 Debye |
| Point Group | C2h |
| Calculation Time | 5 min 27.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000027 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000105 0.001800 YES
RMS Displacement 0.000034 0.001200 YES
Predicted change in Energy=-5.124000D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -565.5351 -0.0005 -0.0003 -0.0002 21.8453 27.2488
Low frequencies --- 39.6940 194.4977 267.9692
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -565.5351 194.4977 267.9361
The calculation was converged and the optimisation was successful.
There was 1 imaginary frequency observed, indicating that it is a transition state.
Discussion
- Temperature Corrections and Energies for Boat Conformer
Zero-point correction= 0.140751 (Hartree/Particle) Thermal correction to Energy= 0.147086 Thermal correction to Enthalpy= 0.148030 Thermal correction to Gibbs Free Energy= 0.111341 Sum of electronic and zero-point Energies= -234.402342 Sum of electronic and thermal Energies= -234.396008 Sum of electronic and thermal Enthalpies= -234.395063 Sum of electronic and thermal Free Energies= -234.431752
- Temperature Corrections and Energies for Chair Conformer
Zero-point correction= 0.142054 (Hartree/Particle) Thermal correction to Energy= 0.147975 Thermal correction to Enthalpy= 0.148919 Thermal correction to Gibbs Free Energy= 0.113169 Sum of electronic and zero-point Energies= -234.414929 Sum of electronic and thermal Energies= -234.409008 Sum of electronic and thermal Enthalpies= -234.408064 Sum of electronic and thermal Free Energies= -234.443814
- Comparison of Energies
|
Optimisation Method |
HF/3-21 | B3LYP/6-31* | Experimental |
|---|---|---|---|
| Chair | 0.072934 a.u.
45.7667 kcal/mol |
0.054284 a.u.
34.0637 kcal/mol |
33.5 ± 0.5 kcal/mol |
| Boat | 0.088610 a.u.
55.6036 kcal/mol |
0.066863 a.u.
41.9571 kcal/mol |
44.7 ± 2.0 kcal/mol |
From the table, it can be seen that the result from B3LYP/6-31G* optimised molecules is much closer to the experimental value than HF/3-21. The error 6-31G* value is about 6-31G* is about ± 2.0 to 3.0 kcal/mol while the error of result from 3-21G could be more than ± 10.0, which is essentially about 30% error. This shows that 3-21G is very basic and is not suitable for comparing energy as normally the energy difference is small, which makes percentage error extremely large and the result being very inaccurate. It is best used as the first step of optimisation to reduce the time for calculation when using higher basis set. 6-31G* is a more accurate basis set which produces more acceptable results.
Mini Project: Diels-Alder Reaction
HOMO and LUMO of cis Butadiene
cis butadiene |
The HOMO and LUMO of the cis butandiene are examined in this section. The cis butadiene was first optimised using semi-empirical AM1 orbital method, the MO was then obtained by choosing 'visualise' in 'MOs' window.
Optimisation Set-up
- Method: AM1
- Basis set: ZDO
- Job Type: Opt+Freq
- Keywords: # opt freq am1 geom=connectivity
Results
The log file of optimised cis butadiene is available here.
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Final Energy | 0.04879718 a.u. |
| Gradient | 0.00001426 a.u. |
| Dipole Moment | 0.0414 Debye |
| Point Group | C2v |
| Calculation Time | 11.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000025 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000440 0.001800 YES
RMS Displacement 0.000182 0.001200 YES
Predicted change in Energy=-8.726240D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -39.9774 -5.7255 -3.4275 -2.2358 0.0010 0.0468
Low frequencies --- 0.0640 312.4536 485.1741
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -39.9773 312.4536 485.1741
The calculation was converged and the optimisation was successful.
The presence of 1 imaginary frequency indicated that this is a TS.
Discussion
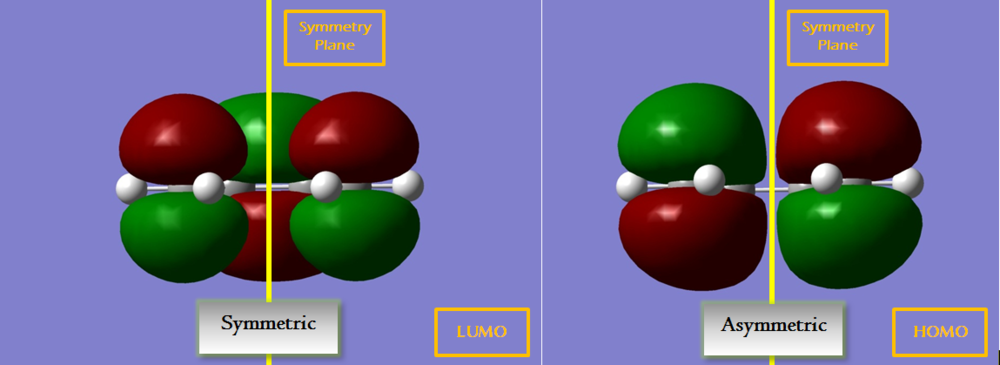
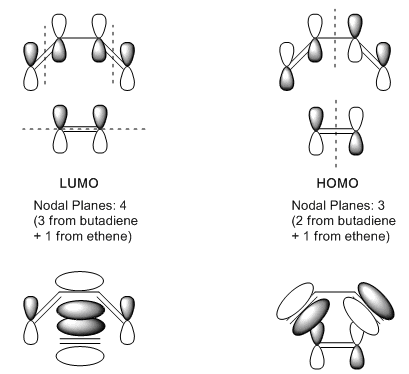
The HOMO and LUMO of the optimised cis butadiene are examined.
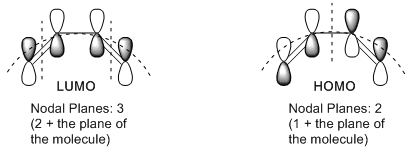
The HOMO is asymmetric and the LUMO is symmetric. Both orbitals are anti-bonding π orbitals. However, HOMO is lower in energy as it has only 2 nodal planes while the LUMO has 3.
The nodal planes are more clearly explained with the diagram below
Diels-Alder Reaction between Ethene and Butadiene
Ethene Butadiene |
The transition state of the Diels-Alder reaction between cis butadiene and ethene is examined by optimising a bent cyclohexane like structure, which is highly similar to the proposed TS of the reaction. The HOMO is also obtained and analysed.
Optimisation Set-up
- Method: AM1
- Basis set: ZDO
- Job Type: Opt+Freq (TS (Berny))
- Additional Keywords: Opt=NoEigen
- Keywords: # opt=(calcfc,ts,noeigen) freq am1 geom=connectivity
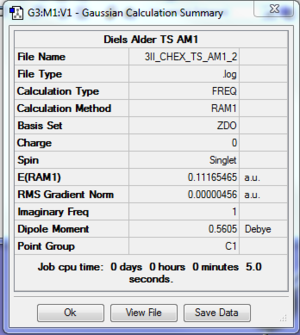
Results
The log file of optimised cis butadiene is available here.
- Summary Table
| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Final Energy | 0.11165465 a.u. |
| Gradient | 0.00000456 a.u. |
| Dipole Moment | 0.5605 Debye |
| Point Group | Cs |
| Calculation Time | 5.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000014 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000074 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-1.183591D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -956.1947 -1.6857 -0.0570 -0.0032 0.0181 2.3564
Low frequencies --- 2.6197 147.2713 246.6380
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -956.1947 147.2713 246.6380
The calculation was converged and the optimisation was successful. The presence of 1 imaginary frequency indicated that this is a TS
Discussion
- HOMO and LUMO
The HOMO and LUMO of the optimised Diels Alder adduct are examined.
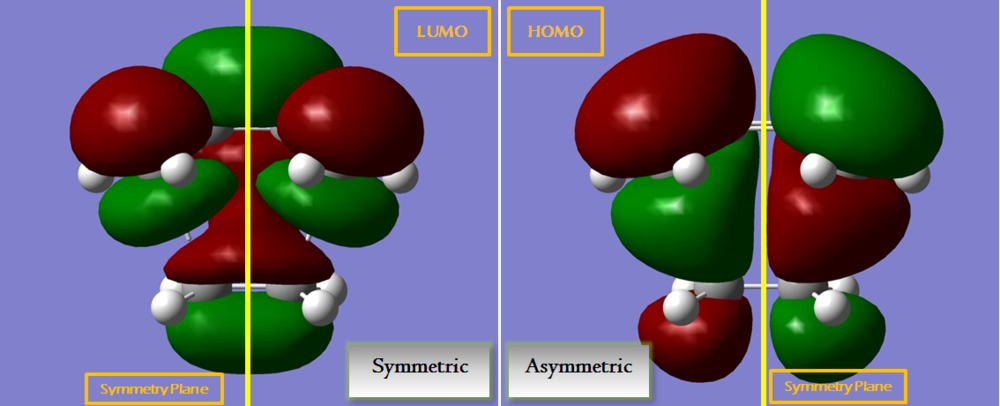
Same as cis butadiene, the HOMO is asymmetric and the LUMO is symmetric. In this case, HOMO has 3 nodal planes while the LUMO has 4.
From the orbital diagram, it can be seen that HOMO forming a ring by joining the π orbitals on the ethene and the terminal π orbitals on the cis butadiene, showing a bonding character between the 2 pairs of terminal carbons. As such, the HOMO could be very significant for the ring forming reaction. On the other side, the LUMO is more similar to a side-on over lap of the π electron cloud, which is not what happened in Diels Alder reaction.
The orbitals of this transition state in the HOMO and LUMO is completely the same as butadiene. Their orientations affect significantly on which bonds are formed and therefore it is very important. In this case, the HOMO of butadiene enables the Diels Alder reaction to take place when it approaches to ethene as its orientation allows the overlap of the C orbitals.
These orbital interactions can be further illustrated with the diagram below.
The lower part of the diagram demonstrated which orbitals were overlapped. As explained above, the orientation of the orbital is very important determining which interactions are formed. The HOMO enables the connection of terminal carbons from butadiene and ethene as the orbitals have the same signs and the interaction is constructive. While in the LUMO, only side on interaction is constructive, the terminal carbon interactions are destructive and nodal space was formed around the green lobe of the butadiene orbital.
- Bond Length
The bond length of the new partial σ bonds are 2.11924 Å and 2.11933 Å. The typical bond length of sp3 carbon is about 1.54 Å and sp2 carbon is about 1.355 Å. Van Der Waals radius of a single carbon 1.7 Å, and for a C-C bond, the value should be 3.4 Å.
It was observed that the bond length of the new partially formed σ bonds are much longer than a single bond, indicating that the bond is still very weak in the transition state. However, the bond is much shorter than the sum of the VDW radius of single carbons. Hence, the partially formed bonds are still attractive, but weaker than a typical normal single bond.
Regioselectivity of Diels Alder Reaction, A Study of Exo and Endo Transition State
The transition state of the Diels-Alder reaction between cyclohexa-1,3-diene and maleic anhydride is examined by optimising a bicyclohexane like structure. Both exo and endo transition states are optimised with the same set-up and the MOs and energies are compared and analysed.
Optimisation Set-up
- Method: AM1
- Basis set: ZDO
- Job Type: Opt+Freq (TS (Berny))
- Additional Keywords: Opt=NoEigen
- Keywords: # opt=(calcfc,ts,noeigen) freq am1 geom=connectivity
Results: exo Transition State
exo TS |
The log file of exo transition state is available here.
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Final Energy | -0.05041984 a.u. |
| Gradient | 0.00000769 a.u. |
| Dipole Moment | 5.5639 Debye |
| Point Group | Cs |
| Calculation Time | 3.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000020 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.001157 0.001800 YES
RMS Displacement 0.000303 0.001200 YES
Predicted change in Energy=-1.360227D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -812.1675 -1.9635 -1.4602 -0.1506 -0.0046 0.9174
Low frequencies --- 1.4610 60.8377 123.8616
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -812.1675 60.8377 123.8616
The calculation was converged and the optimisation was successful. The presence of 1 imaginary frequency indicated that this is a TS
Results: endo Transition State
endo TS |
The log file of endo transition state is available here.
- Summary Table

| File type | .log |
|---|---|
| Calculation Type | FREQ |
| Calculation Method | RAM1 |
| Basis Set | ZDO |
| Final Energy | -0.05150480 a.u. |
| Gradient | 0.00000340 a.u. |
| Dipole Moment | 6.1662 Debye |
| Point Group | Cs |
| Calculation Time | 3.0 s |
- Item Table
Item Value Threshold Converged?
Maximum Force 0.000014 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000337 0.001800 YES
RMS Displacement 0.000059 0.001200 YES
Predicted change in Energy=-1.535554D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -806.3621 -1.9008 -1.5432 -0.5290 -0.0104 0.4252
Low frequencies --- 1.1698 62.4152 111.7336
****** 1 imaginary frequencies (negative Signs) ******
1 2 3
A A A
Frequencies -- -806.3621 62.4152 111.7336
The calculation was converged and the optimisation was successful. The presence of 1 imaginary frequency indicated that this is a TS
Discussion
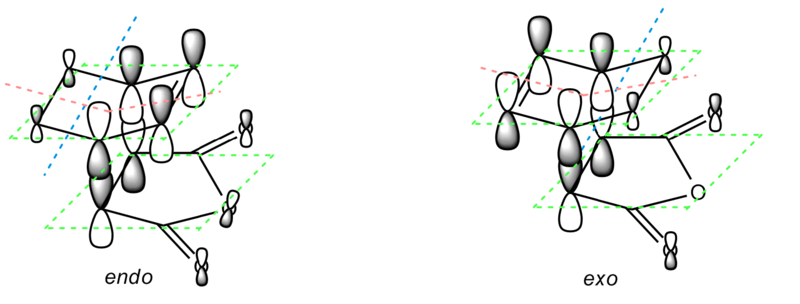
The transition states in this part is much more complicated than in the previous cases. The orbital behaviour is rather complex, especially around O=C-O-C=O bond, as illustrated in the diagrams below. The interaction among the lopes (indicated with thick green dotted lines) make the nodal area around the atom instead of a simple plane. Due to the different orientation, the positions of the nodal planes changed as well. There is a nodal area around a lope on every O atoms at the side. For endo conformer, the nodal area is on top, while for exo conformer, they are below, as illustrated on the second diagram. However, although the orientation changed, the number of nodal planes/areas remains the same which very similar patterns.
The nodal planes on other parts of the molecule is slightly clearer for the rest of the part of the molecule. There are 4 nodal planes, which indicated with blue line, red line and green planes on the first diagram. The lopes below cyclohexadiene and above maleic anhydride overlap to form a constructive π overlap, which is further discussed in the next part.
The last diagram is the HOMO computated from the optimised exo and endo conformers. It is very difficult to see straight the interactions of the orbitals. In order to analyse the orbitals more accurately, the MO diagram with simple lopes without overlap was first produced, as shown in the first 2 diagrams.
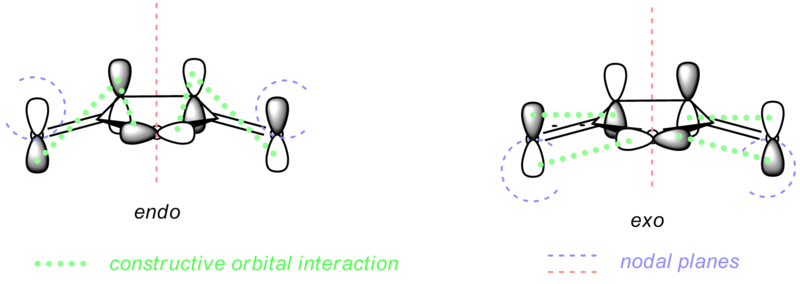 Nodal Planes on O=C-O-C=O bond
Nodal Planes on O=C-O-C=O bond
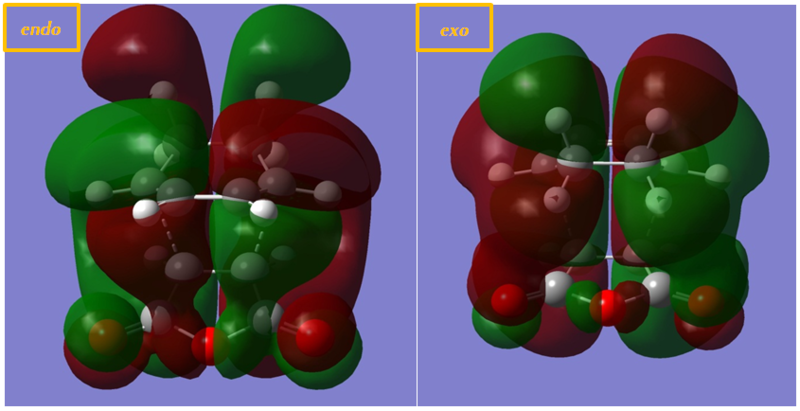
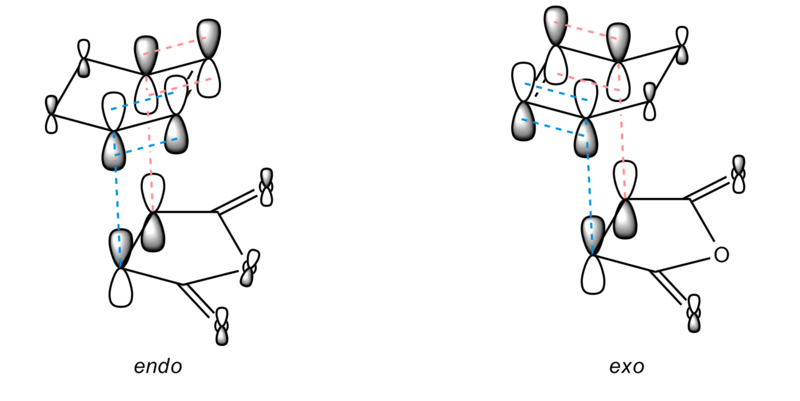
Similar to the reaction between butadiene and ethene, there is secondary orbital overlap which facilitates the reaction. From the diagram below, it can be seen that the π orbitals of the C=C as well as the 4 Cs which are making a new bonds are joined. This would facilitates the reaction as there are additional strong interactions between the C atoms which are forming new bonds.
- Bond length
The bond length of the new bond is shorter in endo conformer, which tells us that the endo transition state is preferable, as its structure is more similar to the product (which bond length would be shorten to about 1.7 Å.) In face, the endo conformer indeed is lower in energy, and hence produce the main product.
In fact, the space between C atom from cyclohexadiene and maleic anhydride in exo is larger. However, the hydrogen atoms on a sp3 carbon would inevitably point down towards the anhydride. Conversely, in endo conformer, the carbon is sp2 hybridised, and therefore the hydrogen is parallel to the anhydride. This makes exo conformer slightly more crowded within the space between 2 reactant molecules.
- Energy Difference
ΔE= -0.05041984 - (-0.05150480)=0.0026936 a.u. = 1.69 kcal/mol endo conformer is about 1.69 kcal/mol more stable than exo. As such, the molecule prefers to react via forming a endo TS, which leads to the main product of this Diels Alder reaction.
Further Discussion
The discussion on transition state to predict the main product is based on the assumption that the reaction is carried out under kinetic condition, which is normally mild with low temperature and short reaction time. This is because a kinetic product is not necessarily the thermodynamically more stable product.
In a chemical mixture, both forward reaction and backward reaction take place. The reaction usually involves 1 or more transition states. The rate determining step involves the highest energy barrier that the reactant has to go over to form the product. The reaction prefers to go via a TS with low energy as less energy is required, the lower the energy, the higher formation rate of this TS would have, which also leads to formation of more products. However, this is also true for the backward reaction. A low energy barrier would mean the product can revert back to reactants easily. At the beginning of the reaction, the amount of product is negligible and hence the reverse reaction is insignificant. Over time, an equilibrium would establish. The product with lowest energy would form, which is the thermodynamic product.
As such, if the reaction is left for a long time under higher temperature, the exo product would form instead, which is more stable in energy.