Rep:Mod:tah1
Part 1
Hydrogenation of Cyclopentadiene Dimer
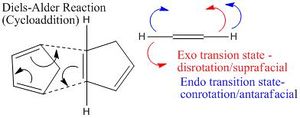
The addition of 2 cyclopentadiene rings is a Diels Alder reaction. This can also be classified as a cycloaddition, the concerted formation of 2 or more σ-bonds. The reaction proceeds via an exo transition state (z-shaped) or an endo transition state (u-shaped).
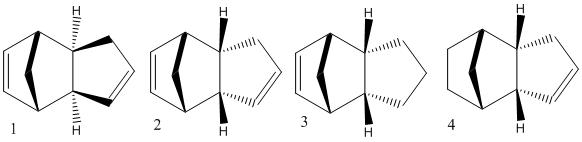
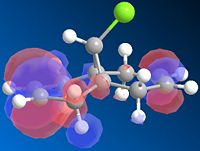
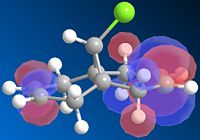
The cyclopentadiene dimer (fig 1&2) with the lowest energy was fig.1 where the bridge adn the pentene ring are pointing up. Here the energy was 31.9kcal/mol. The energy for the endo product where the pentene ring and bridge are on opposite faces had an energy of 34.0kcal/mol. This was not expected as the product formed is fig.2, but this has the higher energy! Normally the exo product would be formed in order to reduce the steric strain on the new bond, it is more strained in the endo product as the pentene ring is pointing on the opposite face to the bridge.
This can be explained however by the orbital overlap of the 2 alkenes in the endo product. During the endo transition state the orbitals on each of the pentene rings are able to overlap with oneanother as they are of the correct symmetry and phase [1]. As this interation is favourable in energy during the transition state the endo product is therefore the major product formed. Due to the transition state in the endo product being most stable and the endo product being formed, it shows that this reaction is kinetically controlled.
Due to the transition state being most like the product than the reactant and that the jump in energy between the exo and endo products is so great this reaction is contorlled kinetically.
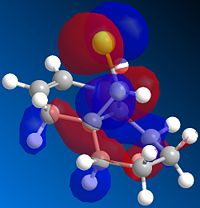
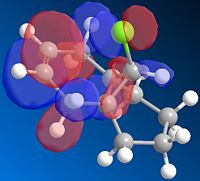
The the lowest energy conformation of the hydorgenation products fig 3 and 4 is product 4, the difference in energy however is not large; 3=35.7kcol/mol, 4=31.2kcal/mol. When comparing the Stretching (3=1.2659; 4=1.1034) and Bending(3=19.8063; 4=14.5151)product 4 came out lower. However when comparing torsion (3=12.2; 4=12.5) Van der Waals (3=5.8; 4=4.5) and H-bonding (3=0.16; 4=0.14) the numbers were even closer to one another. Product 4 ends up with lower strecthing, bending, VdW and H-bonding characters whereas product 3 only has a lower torsion.
From looking at the models the alkene on the pentene ring looks easier to hydrogenate due to there being a clear plane on either side of the ring. On the hexene ring the alkene may be hindered in hydrogenation from the bridge methyl group.
Therefore product 4 is the major product of the reaction and as product 4 is slightly more stable than 3 this reaction is thermodynamically controlled.
1. Clayden, Greeves, Warren and Worthers Organic Chemistry p916
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue).
The final structure of the reaction 5 to 6 had the carbonyl group on the same face as the group added to the pyridinum ring. This is due to the the carbonyl group forming an ionic bond with the metal (in the grignard reagent) and the metal in turn directing the alkyl group onto the pyridine ring and on same face as the carbonyl1. When the wedged group in the heptane ring was pulled to the opposing face of the carbonyl group and the minimisation was calculated the carbonyl group still ended upon the same face as that wedged group. Therefore when the Grignard is added the face which the alkyl group is added to always has the same wedge as the wedged group in the heptane ring. This can also be discribed as the amide bond pushing the carbonyl onto the front face and the nitrogen onto the back face, resulting in the "wedged methyl group".
The energy of structure 5 was found to be 26.3kcal/mol, the product,6 was 28.9kal/mol. Whilst experimenting and forcing the newly added methyl group onto the opposite face the energy was only 28.2kcal/mol. This shows the directing power of the carbonly group as there is not an energy barrier for the methyl group to add to the opposite face, and yet the predominant product formed is that of the methyl on the same face as the carbonyl.
Pentahelicene |
The reaction of 7 to 8 is similar to 5 to 6. The carbonyl group hydrogen bonds with the -NH2 group on the phenyl which directs the nitrogen onto the same face as the carbonyl. Due to the carbonyl group being pushed onto the front face and the nitorgen in the ring being pushed back, the methyl substituent is pushed forwards. This means that the face which the PhNH2 group is added to is always the same as the methy group. These reactions give complete stereochemistry due to the "anchoring step" (when the carbonyl group directs which face to put the new group on)2.
Pentahelicene |
The energy of 7 was found to be 15.8kcal/mol. This is much lower than 5 due to the increased aromatic character of the molecule. The product 8 had an energy of -14.8kcal/mol, this is lower than the starting product and has more aromatic character.
The simple models could be improved by adding wedges to the carbonyl group to show which face it is on
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928.DOI:10.1016/j.tetasy.2004.11.004
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Structures 10 and 11 are atropoisomers, this means that the ring is conformationally locked and therefore they cannot twist into one another.
Structure 10 was found to have an energy of 48.9kcal/mol:
Pentahelicene |
Structure 11 had an energy of 41.8kcal/mol
Pentahelicene |
There is a difference in the enrgies shows that structure 11 is the most stable conformation. However it is hard to see the difference in the structures themselves.
Structure 11 would be exected to be the most stable conformation as there is the chance for hydorgen bonding within the ring causing an increase in stabelisation.
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
How one might induce room temperature hydrolysis of a peptide
Peptide bonds are normally made in the body under acidic conditions with the help of proteases. This reduces the time of hydrolysis dramatically from 500years to less than 1 second.
1-ethylamido-8-hydroxydecahydroquinoline(13) and 1-ethylamido-8-hydroxydecahydroquinoline (14) are conformationally strained isomers with the functional groups to make a peptide bond intramoleculally.
The first structure (13) with the hydrogens forward and the hydroxyl group back gave and energy of 19.3kcal/mol when the amide group is axial and 25.1kcal/mol for the amide group equitorial.
Pentahelicene |
Pentahelicene |
The second structure (14) with the hydrogens on opposite faces had 20.8kcal/mol with the amide group axial and 22.0kcal/mol with the amide group equitorial.
Pentahelicene |
Pentahelicene |
In both cases having the amide group axial lowered the energy of the molecule. This would not be expected as nomally big groups would prefer to go equitorial in order to reduce ring strin - from collision with hydrogens.
Each of the conformers hydrogen bonds between the hydroxyl group and the end NH2 group forming a ring this lowers the energy of the molecule.
The product of the reactions includes a new 6 membered ring, this is favourable for the molecule which is why this particular molecule is faster at forming an amide bond than normal.
The structure where the hydrogens are on the same face is prefered due to the hydorxyl group being at the correct angle to attack the carbonyl on the ethylamido group. When the second structure tries to do this it has to be in the equitorial position which is energetically unfavourable therefore this reaction takes much longer1.
- M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP: DOI:10.1021/jo800706y
Part 2
Regioselective Addition of Dichlorocarbene
The 9-Chloro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene diene used in this experiment is a highly selective unsymmetric diene so it only allows addition onto the alkene on the same side as which the chlorine is facing. This is due to the decrease in the desity of the pi-electron cloud at the other alkene1.
The first minimisation ran and the energy was found to be 17.9kcal/mol, the energy measured from HF/STO-3G is -873.6 Hartree/548182.95 Kcal/Mol and from the B3LYP/6-31G(d)method the energy was caculated to be -887.1Hartree. These energies are completely different. In the the difference in the optimised structures is seen when looked down the bond which is shared by the a rings, the alkene carbons on the same ring as the chlorine are pointing further down on the quantum mechanical model than on the classical. It shows that the quantum mechanical approach has taken into account the repulsion of the large chlorine electron cloud with that of the alkene pi-cloud.
The optimisation of the product found the energy to be 29.2kcal/mol for the classical model and -548940.0Kcal/Mol (-874.8 Hartree)for HF/STO-3G and -888.4Hartree for the B3LYP/6-31G(d). Again this is a very big difference between the quantum mechanical model and the classical. Clearly the quantum model is taking in extra parameters.
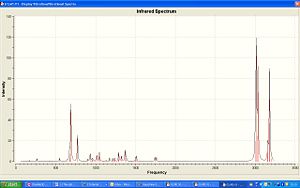 |
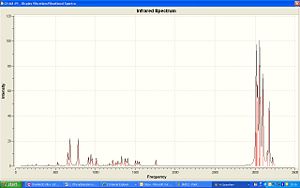
|
| IR of Dichlorocarbene | IR of Hydrated product |
The vibration of the C-Cl bond in the Dicarbene is 772.634cm-1, the hydrogenated product has a high frequency of 782.383cm-1, this shows that the Cl-C bond in this case is more free to move. The Dicarbene has 2 vibrations for the C=C bond, one with the Cl closest to it being a higher frequency, 1760.91cm-1, and the one furthest away being of lower frequency 1740.79cm-1. As the hydrated verision only has one C=C bond there is only one vibrational frequency at 1757.6cm-1.
- Brian Halton, Sarah G. G. Russell J. Org. Chem., 1991, 56 (19), pp 5553–5556 DOI:10.1021/jo00019a015
Structure based Mini project using DFT-based Molecular orbital methods
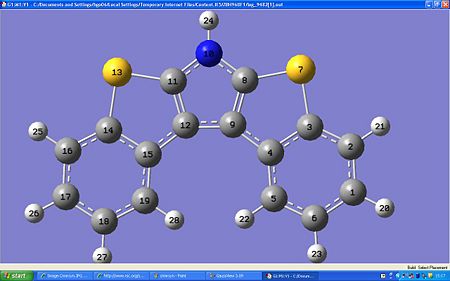
Organic Field-Effect Transistors (OFETs)are transistors made from organic molecules which can control the conductivity of a semi-conductor. This project is looking at the properties of the anti and syn conformers of dibenzothieno[b,d]pyrrole. This molecule is an OFET with sulfur and nitrogen which enhances the properties of the aromatic rings in the solid state and therefore the ability to possibly put onto a circuit board. Another great property is the sulfur-sulfur intermolecular interations, these enhance electron transport between molecules. The paper cited found that the anti-isomer had the best charge transport. Therefore it would be important to distinguish between the 2 isomers in order to get the most effective OFET.
The pathways to make each isomer is different to be stereoselective. However spectra can still be run to confirm products. The 13C NMR will be different due to the 2 carbons closest to the nitrogen have neighbours which are sulfurs in the syn conformation and other carbons in the anti conformer. The same applies to the carbons on the pentene ring furthest away from the nitrogen:
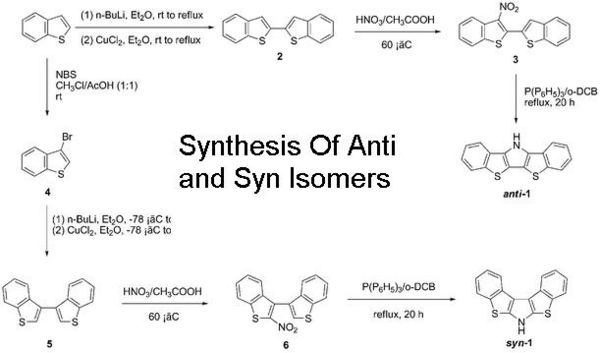
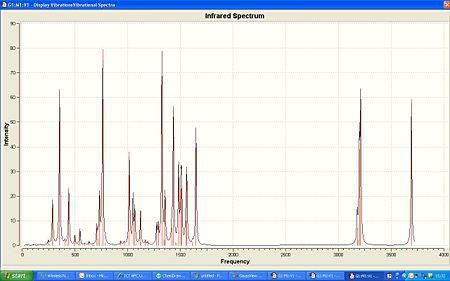
| IR spectra of ANTI | IR spectra of SYN | |
|---|---|---|
 |
|
From the IR differences are meant o be clearly seen. Noticably an IR band for anti at 3414cm-1 for the N-H stretch and for syn a different band at 2920cm-1. However in the IR generated by gaussview no differences are seen apart from the noticable differenc in intensity for the relative peaks - the anti being lower than syn. Gaussview gave 3692cm-1 for the N-H stretch in the anti and 3690cm-1 for the syn. The vibrations are different but not significant enough.
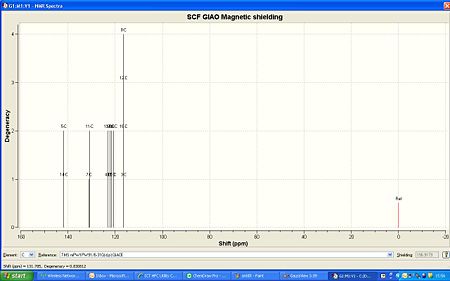
The Anti conformation. Here the sulphurs are on the other side to the nitrogen, and the molecule is planar: The NMR calculated from GaussView is compared with the litriture values (in italics) below;
13C NMR (100MHz CDCl3)δ 141.8 143.324(2C, 14-5), 131.0 129.595(2C,11-7), 123.2 125(2C, 13-4), 122.3 (2C, 17-2), 121.5 120.940 (2C, 19-6), 120.7 (2C, 18-1), 116.4 116.720 (4C, 16 to 12 and 8 to 3).
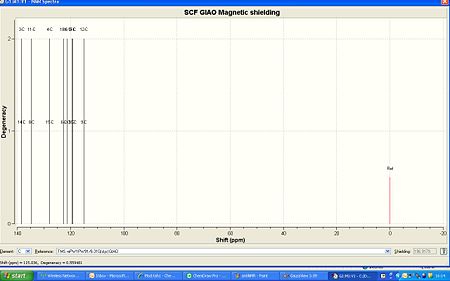
In the Syn conformation the sulphurs and nitrogen are very close to each other, causing this conformation to have a higher energy:
13C NMR (100MHz CDCl3)δ:115.0 120.43 (12-9), 119.3 122.43 (5-19), 119.5 125.39 (1-17), 121.3 125.67 (2-16), 122.5 127.7 (6-18), 127.9 128.61 (4-15), 134.7 129.42 (8-11), 138.4 130.90 (3-14).
The 13C NMR for the anti conformation fitted quite well with the experimental data. On the other hand for the syn it did not and was out by around 5ppm each time, although this was not consistant which shows that the NMRs are quite different in multiplicities too.
Other ways in which Gaussview maybe ablt to distinguish between molecules is using S NMR, here the 2 sulfur atoms have different shits in the anti and syn conformations. 
The isomers also have different decomposition temperatures (286o anti and 235o syn) so it would be easy to distinguish, although then some of the product would be lost!
Anti has a better solubility than syn in ethanol, this could be used to distinguish between the 2 also.
The anti conformation has an energy of -1466a.u. and a dipole of 3.1061Debye:
Pentahelicene |
The syn conformation has an energy of -1466a.u. and a dipole of 0.9358Debye:
Pentahelicene |
The anti isomer is the same energy as syn however it has a bigger dipole, this makes it better for charge transfer in its solid state and so making the anti isomer more desirable.
The spectroscopic methods above show that it is possible to distinguish between the isomers using computational chemistry, however it may not be accurate enough with the experimental values.
- Ting Qi, Yunlong Guo, Yunqi Liu Chem. Commun. 2008 6227-6229 DOI:10.1039/b813683a
- ↑ Clayden, Greeves, Warren and Worthers, Organic Chemistry p916