Rep:Mod:syq9164
Synthesis and computational lab: 1c Jing Sun
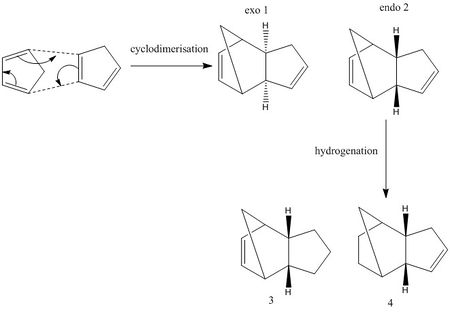
Cyclodimerisation between two Cyclopentadiene
The process of the cyclodimerisation reaction between two cyclopentadiene produces the exo dimer 1 and the endo dimer 2. Endo dimer 2 is favoured over exo dimer 1. In order to understand whether the reaction is thermodynamic controlled or kinetic controlled, Chembio pro 13.0 and Avogadro can be used to calculate the energy information of the two dimer. " MMFF94s" force field was used to optimise the structures. The data will be presented in table A.
| table A Energy type kcal/mol | dimer 1 | dimer 2 | dimer 3 | dimer 4 |
| Bond stretching energy | 3.54300 | 3.46830 | 3.31149 | 2.82311 |
| Angel bending energy | 30.77268 | 33.19005 | 31.93596 | 24.68539 |
| Stretch bending energy | -2.04137 | -2.08243 | -2.10225 | -1.65718 |
| Torsional energy | -2.73112 | -2.94987 | -1.46967 | -0.37832 |
| Out of plane bending energy | 0.01485 | 0.02194 | 0.01318 | 0.00028 |
| Vander waals energy | 12.80173 | 12.35783 | 13.63748 | 10.63719 |
| Electrostatic Energy | 13.01368 | 14.18488 | 5.11950 | 5.14702 |
| Total energy | 55.37344 | 58.19070 | 50.44569 | 41.25749 |
The Hydrogenation of Cyclopentadiene Dimer
By hydrogenation of dimer 2, dimer 3 and dimer 4 are produced.
Analysis
The total energy of dimer 1 is 55.37344kcal/mol. The total energy of dimer 2 is 58.19070kcal/mol. Dimer 1 is lower in energy than dimer 2. So dimer 1 is more thermodynamic stable than dimer 2. However dimer 2 is favoured than dimer 1. Therefore, the cyclodimerisation reaction is kinetic controlled. The angel bending energy contributes to the difference between dimer 1 and dimer 2. The angel bending energy of dimer 1 is 30.77268kcal/mol and of dimer 2 is 33.19005kcal/mol. Because dimer 2 suffers more bonds angel distortion. The graphs show the bond angle between C1,C3 and C22 of dimer 1 is 56.0 degree. the bond angle between C1,C3 and C22 of dimer 2 is 36.9 degree.
| Sturcture | dimer 1 | dimer 2 | dimer 3 | dimer 4 | ||||||||||||
|
|
|
| |||||||||||||
Dimer 4 is more thermodynamic stable than dimer 3. Because the total energy of dimer 3 is 50.44569kcal/mol. The total energy of dimer 4 is 41.25749kcal/mol. The "angel bending energy" and " Vander waals energy" contribute to the total energy difference.
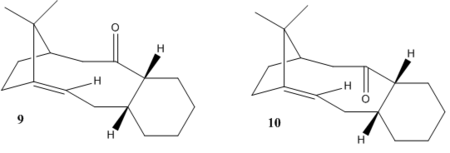
Atropisomers are formed because of the restricted rotation of a single bond. The rotation is restricted because of the steric demand.[1]. During the process of synthesis Taxol, two atropisomers 9 and 10 are formed. In the molecule 9, carbonyl group points up but in the molecule 10, carbonyl group points down. Molecule 11 is synthesized from molecule 9 and molecule 12 is synthesized from molecule 10 after the hydrogenation reaction of alkenes.
| Sturcture | 9 | 10 | ||||||
|
| |||||||
| table B Energy type kcal/mol | intermediate 9 | intermediate 10 | product 11 | products 12 |
| Bond stretching energy | 7.63652 | 7.17041 | 7.25156 | 6.29379 |
| Angel bending energy | 28.28138 | 22.52503 | 25.99149 | 22.78477 |
| Stretch bending energy | -0.07884 | -0.23843 | 0.19663 | 0.28568 |
| Torsional energy | 0.32912 | -1.91757 | 10.68153 | 9.67453 |
| Out of plane bending energy | 0.97697 | 0.66855 | 0.06598 | 0.04411 |
| Vander waals energy | 33.09792 | 31.99787 | 33.72977 | 30.85719 |
| Electrostatic Energy | 0.30697 | 0.83138 | 0 | 0 |
| Total energy | 70.55003 | 61.03724 | 77.91695 | 69.94008 |
Analysis
Same procedures are used in this step. The energy information is in the table B The total energy of molecule 9 is 70.55003kcal/mol. The total energy of molecule 10 is 61.03724kcal/mol. Molecule 10 is less in total energy than molecule 9. So molecule 10 is more stable. Olefinic strain(OS)is the total torsional energy difference between the olefin and hydrocarbon. OS of molecule 9 to molecule 11 is -10.35241kcal/mol. OS of molecule 10 to molecule 12 is -11.5921kcal/mol. They are both smaller than 17kcal/mol. So the carbonyl group is stable in both molecule 9 and 10. Because the OS is small, the double bond is not highly twisted. The consequence is the HOMO-LUMO gap is large. So the reactivity is low[2].
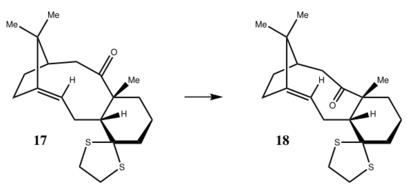
Molecules 17 and 18 are derivatives of molecules 9 and 10. Molecule 17 is more thermodynamic stable than molecule 18. Molecule 17 transfers to molecule 18 after reflux in THF[3]. Simulated 1H and 13C are used to compare with the literature values.
Procedures
Chemdraw3D and Avogadro can be used to create and optimize the 3D structure of molecule 17. HPC files can be generated by calculating "Geometry optimisation" with theory: B3LYP and basis: 6-31G(d,p) in Avogadro. Upload the file to HPC system to get the simulated NMR and energy information.
File:1.mol |
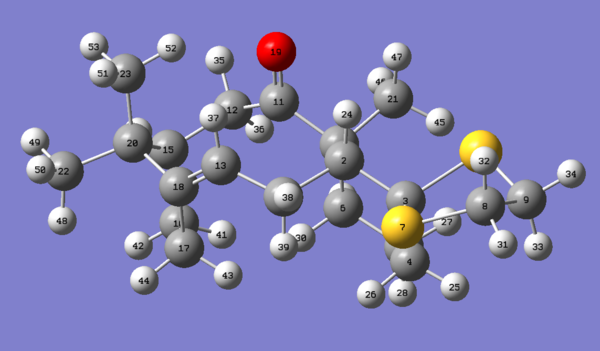
Table C is the energy information of molecule 17. Table D is the information of thermodynamic quantities of molecule 17.
| table C Energy type kcal/mol | molecule 17 | table D | Hartree |
| Bond stretching energy | 15.90499 | Zero-point correction | 0.468397 |
| Angel bending energy | 31.57506 | Thermal correction to Energy | 0.489752 |
| Stretch bending energy | 0.22575 | Thermal correction to Enthalpy | 0.490696 |
| Torsional energy | 11.04354 | Thermal correction to Gibbs Free Energy | 0.422009 |
| Out of plane bending energy | 1.23944 | Sum of electronic and zero-point Energies | -1651.414384 |
| Vander waals energy | 51.99888 | Sum of electronic and thermal Energies | -1651.393029 |
| Electrostatic Energy | -7.20511 | Sum of electronic and thermal Enthalpies | -1651.392085 |
| Total energy | 104.78255 | Sum of electronic and thermal Free Energies | -1651.460772 |
1H NMR[3]
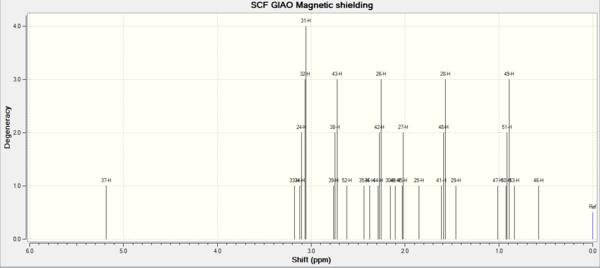
| Shift (ppm) | Degeneracy | Atoms | Literature (ppm) | Atoms |
| 5.18 | 1 | 37 | 4.84 | 1 |
| 3.18 | 1 | 33 | 3.40-3.10 | 4 |
| 3.09 | 4 | 34,24,32,31 | 2.99 | 1 |
| 2.74 | 3 | 39,38,43 | 2.80-1.35 | 14 |
| 2.62 | 1 | 52 | 1.38 | 3 |
| 2.44 | 1 | 35 | 1.25 | 3 |
| 2.38 | 1 | 36 | 1.10 | 3 |
| 2.27 | 3 | 44,42,26 | 1.00-0.80 | 1 |
| 2.16 | 1 | 30 | ||
| 2.10 | 1 | 40 | ||
| 2.03 | 2 | 45,27 | ||
| 1.85 | 1 | 25 | ||
| 1.59 | 3 | 41,48,28 | ||
| 1.46 | 1 | 29 | ||
| 1.01 | 1 | 47 | ||
| 0.91 | 3 | 50,51,49 | ||
| 0.84 | 1 | 53 | ||
| 0.58 | 1 | 46 |
13C NMR[3]
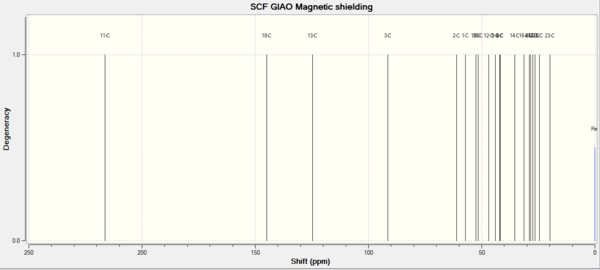
| Shift (ppm) | Degeneracy | Atoms | Literature(ppm) | Atoms |
| 216.32 | 1 | 11 | 218.79 | 1 |
| 144.91 | 1 | 18 | 144.63 | 1 |
| 124.76 | 1 | 13 | 125.33 | 1 |
| 91.40 | 1 | 3 | 72.88 | 1 |
| 61.01 | 1 | 2 | 56.19 | 1 |
| 57.13 | 1 | 1 | 52.52 | 1 |
| 52.51 | 1 | 15 | 48.5 | 1 |
| 51.53 | 1 | 20 | 46.8 | 1 |
| 46.97 | 1 | 12 | 45.76 | 1 |
| 43.92 | 1 | 9 | 39.8 | 1 |
| 42.00 | 1 | 8 | 38.81 | 1 |
| 41.92 | 1 | 4 | 35.85 | 1 |
| 35.48 | 1 | 14 | 32.66 | 1 |
| 31.24 | 1 | 16 | 28.79 | 1 |
| 28.99 | 1 | 6 | 28.29 | 1 |
| 28.41 | 1 | 21 | 26.88 | 1 |
| 27.42 | 1 | 17 | 25.66 | 1 |
| 26.38 | 1 | 22 | 23.86 | 1 |
| 24.60 | 1 | 5 | 20.96 | 1 |
| 19.82 | 1 | 23 | 18.71 | 1 |
Analysis
The simulated H and C NMRS have similar values with literature. The biggest difference occurs in carbon3. In literature, the chemical shift of carbon 3 is 72.88ppm. However in the simulated NMR, the chemical shift of carbon 3 is 91.40. Because carbon 3 connects to two sulfur atoms. It is the spin-orbital error( carbon attaches to heavy elements)
Analysis of the properties of the synthesised alkene epoxides
Shi (molecule 21) and Jacobsen (molecule 24) catalysts[4] has adapted the procedure reported by Jacobsen et al[5],[6] are used in the process of asymmetric epoxidation of alkenes. Shi catalyst is used to epoxidize trans-alkene. Jacobsen is used to epoxidize cis-alkenes. The structures of the two catalysts are shown below:
| Sturcture | Shi | Shi 3D | Jacobsen | Jacobsen 3D | ||||||
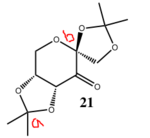 |
|
 |
| |||||||
| Shi | Jacobsen |
 |
 |
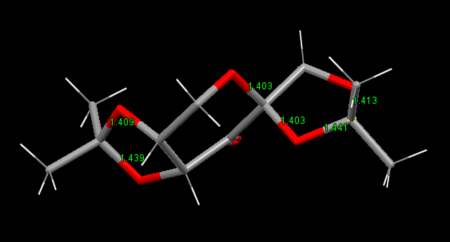
The average value of C-O bond is 143pm. In a O-C-O system of Shi, one C-O bond is larger than 143pm, and the other is less than 143pm. Because electron density of the shorter C-O bond is withdrawed to the longer C-O pi antibonding orbital. In Jacobsen catalyst, electron density is withdrawed by O atoms, so H-H bonds length are different.
The calculated NMR properties of epoxidation products
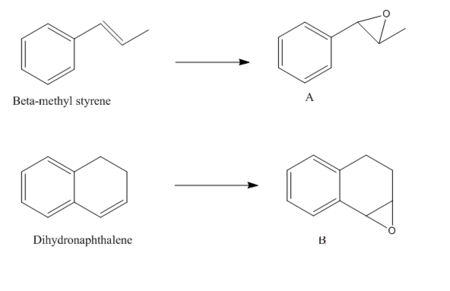
A trans-alkene"Beta-methyl styrene" and a cis-alkene"1,2-dihydronaphthalene" are chose to do the NMR analysis with GaussView and HPC system.
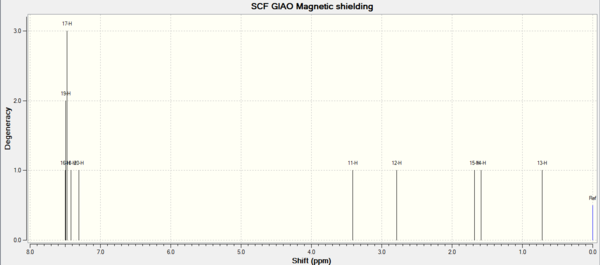
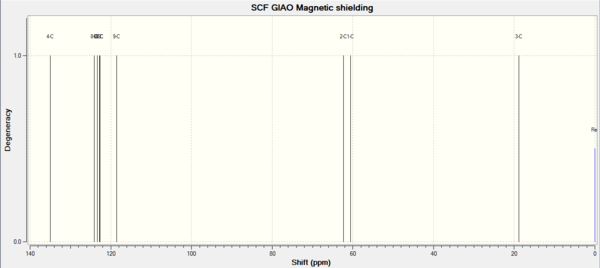
| 1H NMR | 1H NMR | 1H NMR | 13C NMR | 13C NMR | 13C NMR |
| Shift (ppm) | Degeneracy | Atoms | Shift (ppm) | Degeneracy | Atoms |
| 7.49 | 3 | 16,19,17 | 134.97 | 1 | 4 |
| 7.42 | 1 | 18 | 124.07 | 1 | 8 |
| 7.31 | 1 | 20 | 123.33 | 1 | 6 |
| 3.41 | 1 | 11 | 122.80 | 1 | 5 |
| 2.79 | 1 | 12 | 122.73 | 1 | 7 |
| 1.68 | 1 | 15 | 118.49 | 1 | 9 |
| 1.59 | 1 | 14 | 62.33 | 1 | 2 |
| 0.72 | 1 | 13 | 60.57 | 1 | 1 |
| 18.84 | 1 | 3 |
In the H NMR of Beta-methyl styrene, 5 H have chemicalshifts around 7ppm. Because the are in the benzene aromatic system. The H attach to the C=C bonds have chemicalshift around 3ppm. Because they are deshielded by the double bond electrons. The H in methyl group have normal chemical shift around 1ppm. For the same reason, 6 C in benzene have high chemical shift,followed by the C in double bond. The C in methyl group have lowest chemicalshift.
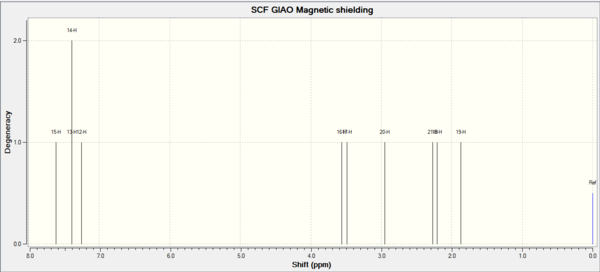
| 1H NMR | 1H NMR | 1H NMR | 13C NMR | 13C NMR | 13C NMR |
| Shift (ppm) | Degeneracy | Atoms | Shift (ppm) | Degeneracy | Atoms |
| 7.62 | 1 | 15 | 135.39 | 1 | 6 |
| 7.39 | 2 | 13,14 | 130.37 | 1 | 5 |
| 7.25 | 1 | 12 | 126.67 | 1 | 4 |
| 3.56 | 1 | 16 | 123.79 | 1 | 2 |
| 3.48 | 1 | 17 | 123.53 | 1 | 1 |
| 2.95 | 1 | 20 | 121.74 | 1 | 3 |
| 2.27 | 1 | 21 | 52.82 | 1 | 8 |
| 2.21 | 1 | 18 | 52.19 | 1 | 7 |
| 1.87 | 1 | 19 | 30.18 | 1 | 10 |
| 29.06 | 1 | 9 |
Same as previous,both H and C which attach to benzene have high chemical shift. The C and H in aliphatic groups have the lowest chemicalshift.

Assigning the absolute configuration of the product
There are three ways to assign the absolute configuration. The first method is to compare with the literature opertical rotation values through Reaxys. Simulated values:
| Beta-methyl styrene | OR | 1,2-dihydronaphthalene | OR |
| S,S | 31.73 deg | R,S | -52.74 deg |
| R,R | -38.29 deg | S,R | 35.87 deg |
Literature values:
| ' | concentration | Enantiometric Excess | solvent | OR | wavelength | temperature |
| 1,2-dihydronaphthalene RS | 0.21 g/100ml | 99 %ee | chloroform | -39 deg | 589 nm | 25 °C |
| 1,2-dihydronaphthalene SR | 0.81 g/100ml | chloroform | -129 deg | 589 nm | 20 °C | |
| Beta-methyl styrene SS | 1.041 g/100ml | chloroform | -47.8 deg | 589 nm | 25 °C | |
| Beta-methyl styrene RR | 0.92 g/100ml | 86%ee | chloroform | 40.8 deg | 589 nm | 25 °C |
The OR value of 1,2-dihydronaphthalene SR molecule in literature is -129 deg. However the value simulated is 35.87 deg. The difference arises from the difference concentration temperature etc. Also optimise the molecule could reduce the error. Generally, the simulated results are similar to the literature results. The energy of the fourth transition state of both RR,SS Beta-methyl styrene have the lowest free energy. So they are the most stable TS. These two TS are chose to calculate k( G=-RTln k). Energy transfers from Hartree to Jouls(1J=2625.5*1000 Hartree)
| Free Energy | SS | RR | difference(hartree) | J/MOL | K |
| TS4 | -1343.024742 | -1343.032443 | 0.007701 | 20218.9755 | 3500.994642 |
The second method is to exam the specific wavelength of rotation with literatures. The third method is to exam the electronic circular dichroism (ECD) and the vibrational circular dichroism (VCD).
Investigating the non-covalent interactions in the active-site of the reaction transition state

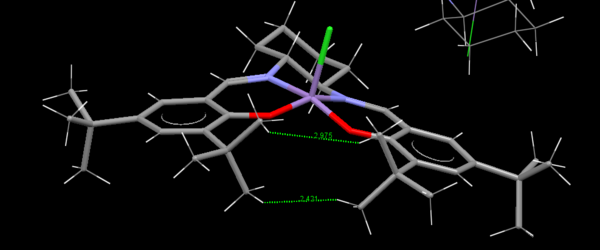
hydrogen bonds, electrostatic attractions and dispersion-like close approaches of pairs of atoms are shown on a NCI[7] graph.Blue colour means attraction, green colour means light attraction,yellow colour means light repulsion and red means repulsion. Most region in this graph is green with slightly yellow, it means the transition state is stable. The red colour occurs in the C-0-C bonds.Because these carbons suffer derivation from sp3 hybirdisition.
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
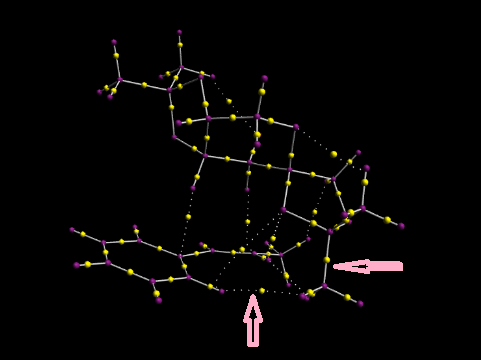
QTAIM exams the electron density in the covalent regions of molecules. The yellow point is the bond critical point.The curvature defines the interaction between atoms. In the graph, the horizontal arrow points to strong interaction between O and H( big electronegativity difference). The vertical arrow points to weak C and H interaction.
Candidate
File:1.mol |
Reaxys could be used to search the candidate. The OR range of searching is >500.
References
- ↑ See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466–5478 (DOI:10.1021/ja00824a025 DOI:10.1021/ja00824a025 ) and 95, J. Am. Chem. Soc., 1999, 121, 3226. DOI: DOI:10.1021/ja990189i . G. ÊIslas-Gonzalez, M. ÊBois-Choussy and J. ÊZhu, Org. Biomol. Chem., 2003, 30-32. DOI: DOI:10.1039/b208905
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
- ↑ 3.0 3.1 3.2 Spectroscopic data: L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283. DOI:10.1021/ja00157a043
- ↑ J. Hanson,J. Chem. Educ., 2001, 78, 1266; DOI:10.1021/ed078p1266
- ↑ E. N. Jacobsen , W. Zhang , A. R. Muci ,J. R. Ecker , L. Deng J. Am. Chem. Soc., 1991, 113, 7063–7064; DOI:10.1021/ja00018a068
- ↑ M. Palucki , N. S. Finney , P. J. Pospisil , M. L. Güler , T. Ishida , and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 948–954; DOI:10.1021/ja973468j
- ↑ J. L. Arbour, H. S. Rzepa, J. Contreras-García, L. A. Adrio, E. M. Barreiro, K. K. Hii, Chem.Euro. J., 2012, 18, 11317–11324, DOI:10.1002/chem.201200547
